A Good Look at Nanocrystals
The chemical reactions that keep sulfur and other pollutants from leaving automobile tailpipes rely on catalysts in the form of microscopic particles dispersed within the large surface area of a porous material. But the atomic-level details of these reactions are hard to study and have never been observed directly. The 31 January PRL contains the first direct views of the catalyst that removes sulfur from gasoline and other fuels. A Danish team managed to disperse the nanocrystals on a surface in a controlled way, and their scanning tunneling microscope (STM) images revealed surprising crystal structures unlike those of larger crystals. The technique demonstrates a new way to study catalysts at the atomic scale.
Refineries reduce the sulfur content in gasoline, diesel fuel, and other oil products, by sending them–together with hydrogen–through a porous material coated with molybdenum disulfide ( ) nanocrystals. The chemical reactions on the nanocrystal surfaces remove much of the sulfur, but governments in Europe and the U.S. are pressing for drastic reductions in the sulfur content of fuels over the next several years to enhance air quality.
To improve the process, researchers need to understand it in detail, but despite decades of research, many questions–such as the precise locations of the chemically active sites–have remained uncertain. “People were just guessing; there were no direct observations,” says Flemming Besenbacher of the University of Aarhus in Denmark. Part of the problem was that on a clean surface the 40-angstrom-wide nanocrystals tend to clump together into larger crystals with different properties.
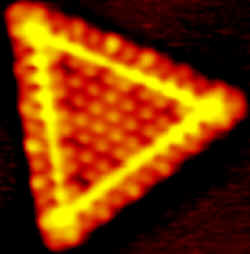
To keep the nanocrystals dispersed, Besenbacher and his colleagues used gold as a template, because its surface structure contains a periodic array of sites that attract small clusters of Mo atoms. They converted the Mo clusters to nanocrystals by exposing them to gas. Surprisingly, the crystals were one-atom-thick triangles, whereas larger crystals normally have hexagonal surfaces. The team surmised that the differences in chemical properties between single-layer and multilayer nanocrystals observed by others may be explained by the two different structures.
Indirect evidence suggested that the reactions in this and other similar catalysts occur at edge defect sites (where atoms are missing from the structure), rather than on the crystal planes, so the researchers took special interest in the edge structure. They found slightly rearranged, or “reconstructed,” rows of sulfur atoms along the triangle edges. The team was able to create single-atom defects in these rows using atomic hydrogen gas, and in future work they hope to study the catalytic reactions that aught to take place there.
“There were many attempts to understand the mechanism of this reaction,” says Gerhard Ertl of the Fritz Haber Institute in Berlin, but this is the first to reveal the reaction sites in atomic detail. He explains that other STM studies have looked at catalytic reactions on metallic surfaces. is nonmetallic and contains two different types of atoms, which makes the reaction more difficult to analyze. Ertl cites a host of related catalysts used in the chemical industry that can benefit from the new methods, and he is certain the results will help researchers learn how to take more of the sulfur out of oil-based fuels.


