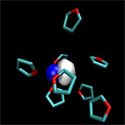
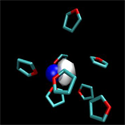
One molecule at a time
With so many atoms and molecules to keep track of, it’s not surprising that the process of dissolving one substance in another is difficult to describe. A simplifying assumption is therefore that the solvent is a continuous medium and that the solvation of an atom or molecule occurs smoothly in time.
Writing in Physical Review Letters, Arthur Bragg and Benjamin Schwartz at the University of California, Los Angeles, and William Glover, now at Stanford University, both in the US, present sensitive measurements that show how this picture breaks down. They use ultrafast spectroscopy and quantum molecular dynamics simulations to track the solvent molecules surrounding a sodium atom as it dissolves in a room-temperature organic liquid and show that the motion of even a single solvent molecule moving in the vicinity of a sodium atom is enough to have pronounced effects on how the sodium atom absorbs light.
Bragg et al.’s measurements clearly identify the sequential arrival of individual solvent molecules in the first solvent shell around the atom, showing that solvent molecules rearrange around dissolving atoms in discrete steps.
The results help explain why the rate of solvent relaxation depends on the initial state of the system—namely, the specific configuration of the organic molecules around the sodium atom—and how using a continuum description of molecular relaxation can miss key elements of the solvation process. – Deniz van Heijnsbergen