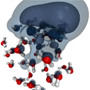
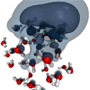
Electrons in hot water
When radiation kicks an electron from a water molecule, the ionized molecule effectively robs a proton from a neighboring molecule, leaving an radical that, in a cellular environment, can be harmful to DNA. While scientists are understandably interested in modeling the dynamics of the chemistry of radicals, there is also substantial experimental and theoretical work aimed at following the path of the dissociated electron in an aqueous environment.
Measurements of the localization of the dissociated electron have mostly been performed on cold clusters of water, while simulations have mainly been limited to clusters of a few tens of molecules. Writing in Physical Review Letters, Ondrej Marsalek and colleagues at the Academy of Sciences of the Czech Republic, in collaboration with researchers in Germany, explore the potential pitfalls that come from assuming the excess electron’s path in a small cluster of water molecules can be extrapolated to the true “bulk” liquid case.
Marsalek et al. apply molecular dynamics to predict the trajectory of an excess electron in a neutral cluster of thirty-two water molecules. Simulations of a room-temperature cluster show the electron initially hovers near the surface of the cluster before it is trapped in a cage of polarized water molecules after about picoseconds. In colder clusters the same electron trapping process can take much longer or the electron becomes trapped in a metastable state.
The central message is that to extrapolate to bulk water from cluster calculations, simulations must be performed in the “warm” regime, namely, a temperature range fairly close to room temperature. – Jessica Thomas