Training Catalytic Atoms to Stop Fidgeting
Much of modern industrial chemistry relies on catalysts to drive useful reactions toward desired end products and increased yields. What makes these catalysts successful becomes clearer only with the ability to identify and analyze individual atoms on oxide surfaces, so having model systems to explore the chemistry and physics of surfaces is crucial. In a paper in Physical Review Letters, Zbynĕk Novotný and colleagues at the Vienna University of Technology, Austria, report their development of thermally stable arrays of gold atoms on iron oxide that may be ideal for answering key questions in catalysis.
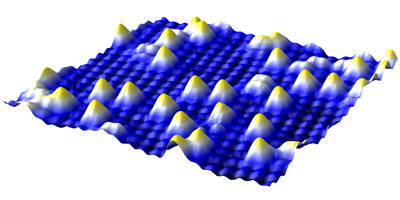
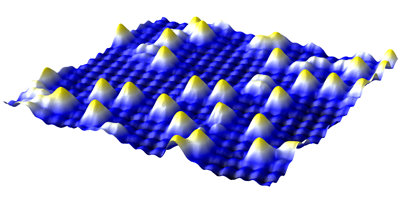
Previous work has hinted at a size effect in catalysis: as clusters of catalytic metal atoms get smaller and smaller, they become more chemically active. Taken to the limit, individual atoms may be the most active, but studying this behavior demands a stable, well-characterized combination of atom and surface. Typically, however, gold atoms are highly mobile on these kinds of substrates and researchers have to cool them to cryogenic temperatures to hold them steady, making investigation under realistic conditions difficult.
Novotný et al. find that gold atoms sit comfortably on single crystals of (magnetite) cut to present a particular surface structure at temperatures as high as C. Owing to charge ordering in the iron oxide, the surface acquires a lateral electronic structure that may stabilize the gold atoms, along with several other adsorbed atoms studied by the researchers. This suggests that the team has discovered a model system that may be universally applicable to detailed investigations of small cluster catalysis under actual reaction conditions. – David Voss