Classical vs Quantum
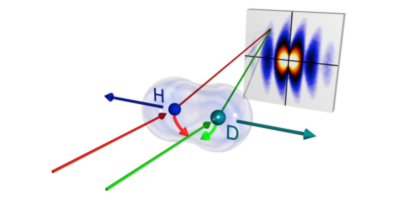
A particle passes through a single slit and then through one opening of a double slit. Can the pathway of the particle be determined without destroying the interference structure? This was a question first debated by Einstein and Bohr as they tried to understand the newly developed ideas of quantum physics. Einstein argued that classical physics was sufficient; you could determine the particle’s path by measuring the momentum transfer imparted from the deflection of the particle by the first slit. Bohr claimed instead that the slits, as well as the particle, behave as quantum objects, whose position and momentum are uncertain—we can either know which path the particle takes through the slit maze, or how long the path is, but not both.
So who was right? Writing in Physical Review Letters, Lothar Schmidt and colleagues, from Goethe University in Germany, show that Bohr was right. In their experiments, the team replaced the slits with hydrogen-deuteron molecular ions and bombarded them with helium atoms. As the atoms collided with the ions, an electron was exchanged between the atom and the ion. By measuring this exchange they could determine the positions and orientation of the atoms and ions. The scattering of the atoms was consistent with Bohr’s view; you need a quantum description of the slits and particles to understand the results. However, the results could still be correctly predicted using classical slits, but only if the particle simultaneously passed through both holes of the double slit and transferred half of its momentum to each path. – Katherine Thomas