Liquid to Liquid
The most familiar phase transitions of water are when it freezes into a solid or evaporates into a gas. But water, like a small handful of other liquids, can also exist in multiple liquid states, which are distinguished by their density and the local order of the molecules. Such liquid-liquid phase transitions are rare, and finding new ones would allow scientists to probe the fundamental nature of the liquid state. Writing in Physical Review Letters, Hajime Tanaka, and co-workers from the University of Tokyo, Japan, present optics experiments showing that triphenyl phosphate (TPP)—a material commonly used as a flame retardant—exhibits a liquid-liquid phase transition.
Researchers have previously shown that supercooling TPP (that is, quickly cooling it to a temperature below which it would normally freeze) leads to density changes that could be signs of a liquid-liquid phase transition. But small nanocrystals were also observed to form upon supercooling TPP, suggesting that instead of undergoing a liquid-liquid phase transition, TPP might actually be freezing, albeit very slowly.
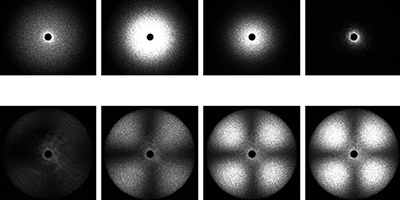
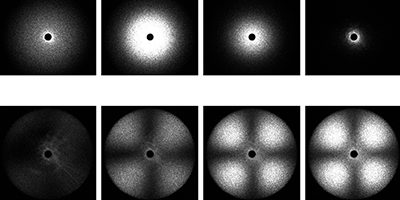
To separate the two phenomena, Tanaka and co-workers supercooled TPP to various temperatures below its freezing point and used polarized and depolarized light to measure the liquid’s refractive index (which is sensitive to the liquid density) and optical anisotropy fluctuations (which track the formation of nanocrystals) over time. The researchers saw evidence for both density changes and nanocrystal formation upon cooling. But their key finding was a temperature at which isotropic density fluctuations first increased and then decreased over time—a possibility that can’t arise from crystal formation and therefore likely arises from a true liquid-liquid transition. The results suggest that while crystallization and the liquid-liquid phase transition occur simultaneously, they are in fact different phenomena. – Katherine Wright