Bubbles Pop, Droplets Don’t
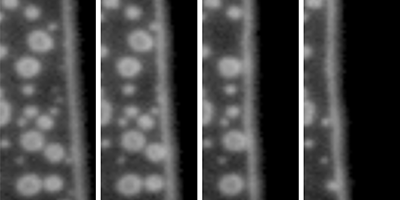
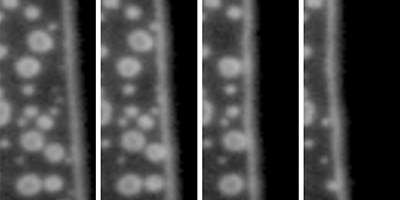
Nanometer-sized bubbles, which can form on the surface of submerged objects, could be used for drag reduction in micro- and nano-fluidic devices. These nanobubbles are surprisingly stable—living for up to days (see Synopsis: Can’t Burst This Bubble) but are notoriously hard to identify and distinguish from other, similarly sized contaminants on an object’s surface. Now Claus-Dieter Ohl and his group at Nanyang Technological University, Singapore, have developed a method that can accurately differentiate between nano-sized bubbles, liquid drops, and solid particles on a water-covered surface by observing how the different objects responded as water was displaced from their surfaces.
The authors prepared liquid-filled microchannels covered with nanobubbles, liquid polymer drops, and solid particles. Air was then slowly pumped in, displacing the liquid and progressively uncovering the channel surface. The group used total-internal-reflection microscopy—an imaging technique with subwavelength resolution—to monitor how each contaminant behaved when reached by the moving air-water interface. If no contaminants were present, the interface moved smoothly as air pushed the water back. When particles or drops were encountered, the interface became temporarily stuck, before jumping over the contaminant. Bubbles, on the other hand, merged with the moving interface, shrinking, and then bursting. Using a high-speed camera to resolve the collapse of the bubbles, Ohl et al. found that they shrank more slowly than current models predict. The authors suggest that this slow popping may arise from molecular-level interactions of the water in contact with the bubble surface. Their technique provides robust criteria to confirm the gaseous nature of a contaminant on a water-submerged object, albeit only by destroying the bubbles.
This research is published in Physical Review Letters.
–Katherine Wright