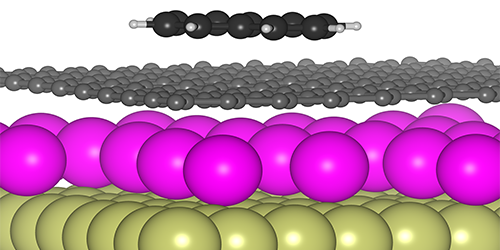
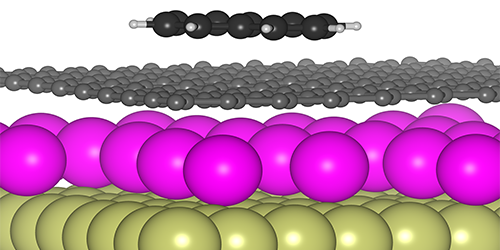
A van der Waals Tuning Knob
The van der Waals attraction occurs when two objects come close enough to induce an electric polarization in one another. Researchers have now demonstrated a way to tune the van der Waals force exerted by graphene on a molecule. The technique, which is based on doping the graphene from the back side, could be used to control adsorption to graphene for a wide range of molecules and raises the prospect of electrical control of adsorption.
Tunability is nothing new to graphene. Its unique cone-shaped electronic band structure allows one to control the potential energy (or Fermi level) of its electrons by simply adding a gate electrode or dopant atoms. Recent work has used this Fermi-level tuning to manipulate the strength of ionic bonding between atoms and the graphene surface. Felix Huttmann from the University of Cologne, Germany, and his colleagues have extended this surface control to the weaker, but more general, van der Waal interactions.
The researchers produced a graphene sheet on a metal substrate and then added either electron-donor atoms (n-type dopants) or electron-acceptor atoms (p-type dopants). In both cases, the dopants nestled between the graphene and metal, leaving a “clean slate” on the top surface of the graphene. To explore van der Waals interactions, the team exposed the graphene to naphthalene molecules and observed their adsorption on the surface with scanning tunneling microscopy. When the samples were heated, the temperature at which naphthalene desorbed was higher for n doping than for p doping, implying that the van der Waals attraction was stronger in the former. Theoretical calculations confirmed this picture by demonstrating that n doping causes the electron orbitals around carbon atoms to extend out further spatially, making the atoms more easily polarizable.
This research is published in Physical Review Letters.
–Michael Schirber