Mind the Interface
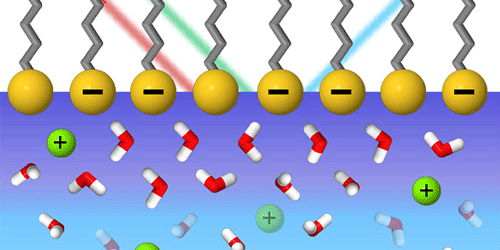
The interfacial layer between water and a charged surface can have a rich chemistry in which water molecules and ions interact via hydrogen bonding and electrostatic forces. Such interfaces are ubiquitous in nature and play a key role in energy-conversion applications, such as water splitting for fuel cells. Determining the exact chemical structure of a charged interface layer is, however, challenging with most probes because the signal from the interface is entangled with that of the underlying bulk water layer. Now, researchers have developed a new spectroscopic technique that extracts information about the interface layer by carefully separating out the signal from the bulk layer. This technique can be used to reveal the molecular structure of complex biological or electrochemical interfaces.
Chuanshan Tian, at Fudan University in China, and his colleagues prepared a single-molecular layer of lignoceric acid on top of water, creating an interface with varying amounts of surface charge. Using lasers, the team measured the combined molecular vibrational signal from water molecules—in the bulk layer and in the interface layer—and from the lignoceric acid molecules. The researchers were able to separate out the bulk signal contribution by characterizing, for the first time, a second-order optical property of water that had been previously known but never quantified. With this approach, Tian and his team were able to measure the geometry and strength of the molecular bonds in the charged interface layer. They showed that the molecular structure in this region depends strongly on the pH and ionic makeup of the surrounding medium.
This research is published in Physical Review Letters.
–Katherine Kornei