Water Under Confinement
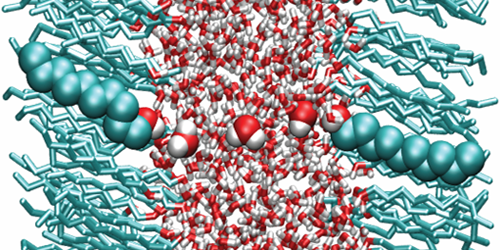
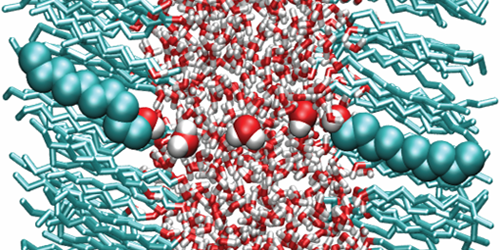
Because of its polar nature, water has a large dielectric permittivity ( 𝜖=80). Such a value means that Coulomb interactions between charged particles are reduced by a factor of 80 compared to vacuum—a property that affects the behavior of ions or proteins in aqueous solutions. But new calculations suggest that water’s dielectric properties may be significantly altered when the liquid is confined to ultrathin layers. This finding will help scientists understand biological processes occurring in subnanometer water layers, such as ion dynamics in the tightly stacked membranes of certain photosynthetic complexes.
In many situations, water can be modeled using continuum theories that assume the liquid has the permittivity of the bulk. However, many experimental and theoretical studies hinted that this assumption might break down when water is confined to the nanoscale. Alexander Schlaich at the Free University of Berlin, Germany, and colleagues carried out a systematic study of such confinement effects. Using molecular dynamics simulations, the authors calculated the permittivity of water layers confined between two polar surfaces as a function of layer thickness.
The results show that water slabs thicker than 1 nm have the same permittivity as bulk water. For smaller thicknesses, however, the permittivity becomes highly anisotropic, with a perpendicular and a parallel component (describing dielectric effects on electric fields parallel and perpendicular to the slab plane, respectively). While the parallel component is slightly larger than that of the bulk, the perpendicular one is reduced by almost 1 order of magnitude for thicknesses below about 0.5 nm. The simulations suggest this asymmetry is caused by molecular interactions between water and the polar surfaces, which enhance the alignment of the water dipoles parallel to the plane but reduce that of the perpendicular dipoles.
This research is published in Physical Review Letters.
–Matteo Rini
Matteo Rini is the Deputy Editor of Physics.