Probing Cell Squishiness
Biological cells stretch and squeeze when crawling along a surface, when sensing their environment, or when dividing into two cells. A new technique based on atomic force microscopy (AFM) makes it possible to track the changes in mechanical properties of a living cell. The AFM probe vibrates in the liquid surrounding the cell, and the probe's driving mechanism need not be submerged. As a demonstration, the researchers mapped the elastic responses of human cancer cells, revealing the way in which stiffening and softening of a cell is associated with moving and reproducing.
Cells are squishy bags of fluid, but the squishiness—or elasticity—is not uniform across the cell, nor is it constant over time. For example, cells can control their stiffness and shape by forming and dissolving protein-based filaments. Experiments also suggest that elasticity could be an indicator of disease, as cancer cells appear to be softer than normal cells [1]. Mechanical studies have typically relied on AFM to test the responses of individual cells. However, the standard AFM technique, which involves a sharp, needle-like probe extending down from a vibrating cantilever, is not completely compatible with the cellular environment. “Studying elastic properties of cells means dealing with water,” says Penger Tong from the Hong Kong University of Science and Technology. Submerging the cantilever leads to friction with the liquid, making it difficult to keep the device vibrating at a consistent rate.
Tong and his colleagues have developed an AFM technique that avoids these difficulties by keeping the cantilever in air. To be able to reach cells in solution, the team uses a probe that is about 150 micrometers long, which is roughly 10 times longer than the usual AFM probe. Also unusual is the probe material. The team chose glass, rather than more common metallic materials, because it allowed them to use a coating that limited proteins from adhering to the tip, as well as facilitating the probe fabrication, assembly, and cleaning.
In their experiments, Tong’s team placed human cancer (HeLa) cells on a flat surface and covered them with a water solution whose depth was maintained between 50 and 100 micrometers. The AFM probe was lowered into the fluid layer and was set to vibrate in a combination of two modes simultaneously. The first vibration mode had a frequency of 50 kHz and an amplitude that depended on the distance to the cell. By monitoring this amplitude, the team measured the height of the cell.
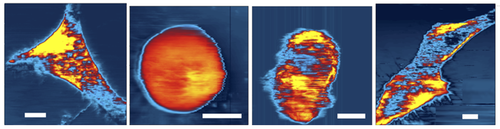
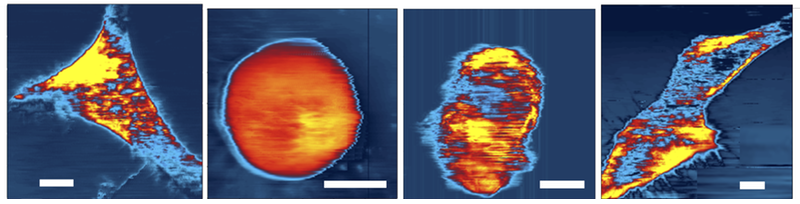
The other vibration mode, with a frequency of 300 kHz, provided information about the mechanical response of the cell. When the probe was less than 100 nanometers from the cell, the water in the gap between the tip and surface couldn’t flow rapidly enough to keep up with these faster vibrations. As a result, this gap fluid became an extension of the probe’s tip, effectively pushing on the cell surface and causing it to deform up and down, like a drumhead. By recording the force needed to maintain resonant vibration of the cell surface, the team could extract the Young’s modulus of the cell, which is a measure of its elasticity. By scanning over the entire surface of the cell, the researchers made elasticity maps.
In some of their trials, Tong’s team was able to capture cells in the process of moving over the surface. The elasticity map showed that the leading edge of the cell became stiffer, in agreement with other observations of chemical reactions associated with cellular movement. “Our maps offer a quantitative description of something we already knew about,” Tong says.
They also were able to witness several stages of cell division, documenting the changes in the mechanical profile as chromosomes were replicated and the two daughter cells pulled apart. Further studies along these lines could reveal the influence of the mechanical environment on the final state of the daughter cells.
Chemical physicist Maxime Dahan of the Curie Institute in Paris says that the work is "a nice combination of advanced instrumental tools and elaborate modeling." He says that the mechanical properties of cells play an essential role in many cell functions, as well as in operations of entire tissues. Previous measurement methods often involved contact with the cells, which risked interfering with their activity. "Here, the fact that you have a noncontact mode is a big advantage," Dahan says.
This research is published in Physical Review Applied.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- S. E. Cross, Y.-S. Jin, J. Rao, and J. K. Gimzewski, “Nanomechanical Analysis of Cells from Cancer Patients,” Nat. Nanotech. 2, 780 (2007).