Detection of Ortho-Para Transition in Molecules
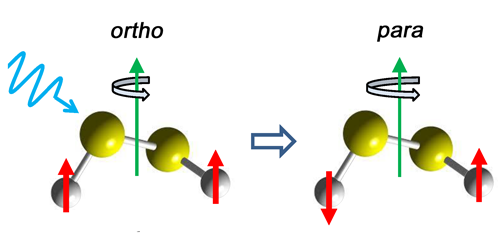
Certain symmetric molecules come in two states, ortho and para, which differ in their nuclear spin alignment. Photon-mediated transitions between these spin isomers are strongly forbidden, but researchers have now observed such transitions for the first time. Studying a molecule with an advantageous mixing of ortho and para states, the team detected the microwave emission from several ortho-para transitions and observed intensities 1000 times smaller than those of allowed transitions.
The most familiar ortho and para states are those of the hydrogen molecule, . At room temperature, about 75% of hydrogen molecules are in the ortho state, with the spins of the two hydrogen nuclei in a symmetric configuration (pointing in the same direction). At lower temperatures, molecular collisions convert most of the ortho- to antisymmetric para- , as it has slightly less energy. However, radiative ortho-para transitions (where a single molecule emits or absorbs a photon) are extremely unlikely, with a predicted spontaneous-emission rate per molecule of less than once per the age of the Universe.
Hideto Kanamori, from the Tokyo Institute of Technology, and colleagues considered disulfur dichloride ( ), a helically twisted molecule with relatively strong hyperfine interactions between its nuclear quadrupole moment and its molecular electric field. As a result, certain rotational states in are mixtures of ortho and para nuclear states. The team showed that, through this mixing, a forbidden ortho-para transition can “borrow” intensity from an allowed ortho-ortho or para-para transition. The team verified this prediction by observing the microwave emission lines that are characteristic of several forbidden transitions. The implied radiative transition rate of an isolated molecule is once every few thousand years.
This research is published in Physical Review Letters.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics based in Lyon, France.