How to Measure Viscosity Inside Cells
The cell nucleus is the headquarters for many molecules, such as proteins and RNA, that carry information throughout the cell. Understanding how these molecules operate requires an understanding of the medium through which they move. Now researchers have developed the first noninvasive technique for measuring the viscosity inside the nucleus. The team used micrometer-sized cellular structures, called nucleoli, to act as natural probes of the nuclear environment and found that the viscosity was about 300 times that of honey, in agreement with other more invasive measurements.
Most cells other than bacteria have a nucleus that hosts the organism’s DNA along with the complex machinery for reading the genetic code in an organized way. The machinery produces messenger RNA strands that travel from the nucleus to the protein manufacturing sites called ribosomes. “These vital cellular processes will be impacted by the material properties inside the nucleus,” says Alexandra Zidovska, of New York University. Previous measurements of nuclear properties have used small beads, which are injected into the nucleus and then observed while being manipulated by magnetic fields. The injection procedure involves a needle that damages the cell, so it is unclear if the measured properties are representative of a healthy, undisturbed cell, Zidovska says.
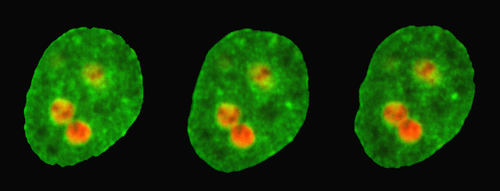
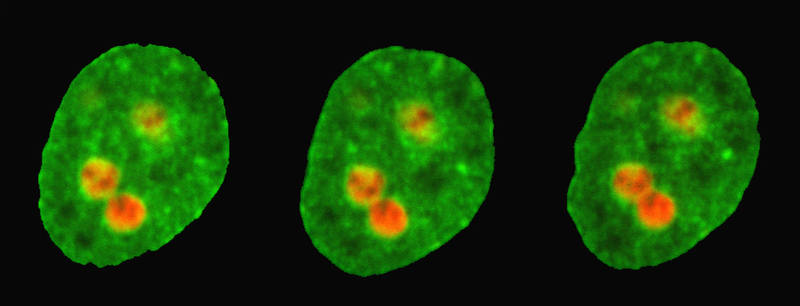
The new method from Zidovska and her team takes advantage of nucleoli, which are like environment-sensitive probes "that nature put in the nucleus for us,” she says. Nucleoli—membraneless, spherical structures made up of RNA and proteins—play a central role in the synthesis of ribosomes. By observing the movements of the nucleoli with a high-resolution microscope, the team could extract the properties of the nuclear environment. But in order to analyze these motions, the researchers first had to characterize some of the nucleoli’s intrinsic properties.
The team performed experiments on cultured human cells that they genetically modified to carry two fluorescent markers: a red marker tagged the nucleoli, while a green marker highlighted the rest of the nucleus. The nucleoli appeared as red circular regions under the microscope, and the researchers were able to spot minute fluctuations on their edges, consistent with previous proposals that the nucleoli behave like liquid droplets, rather than solid aggregates. The amplitude of the surface fluctuations provided a measurement of the surface tension. Surprisingly, they found that the surface tension of the nucleoli was extremely low—about 50,000 times less than that of water droplets in air.
Knowing the surface tension of the nucleoli, the team could then analyze other movements of these structures. Specifically, they developed a method for recording the collision and fusion of two nucleoli, which is a rare event in the lifetime of a human cell and one that had not been directly observed before. The experiments revealed a slow merging process driven by surface tension that took about 15 minutes, which is long for a cellular process. Using a theory that describes how bubbles and droplets coalesce, the team inferred an extremely high viscosity of around 3000 pascal-seconds for the nuclear fluid (honey’s viscosity is about 10 pascal-seconds, while water’s is 0.001 pascal-seconds).
The ability to measure viscosity directly in a living, undisturbed cell could provide a benchmark for identifying the effects of disease on the material properties of cells. Studies have shown that the size and shape of nucleoli can be different in cancer and Alzheimer’s disease patients, compared with healthy subjects. These nucleoli differences suggest that the material properties of the nuclear environment may change as a result of disease, which—if verified by further studies—could offer new information about how certain diseases disrupt cell functioning, Zidovska says.
“We have very few approaches for examining the material properties” in cells, says bioengineer Cliff Brangwynne from Princeton University in New Jersey, so “the approach described in this paper moves the field forward.” He says the work by Zidovska and colleagues is part of a larger effort by many researchers who want to ultimately link the mechanical properties of nucleoli and other cellular components to the processing of genetic information.
This research is published in Physical Review Letters.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.