Bead Chains Impersonate Polymer Molecules
Granular materials such as sand often behave like a collection of molecules, flowing like liquids in some conditions or locking together in solid structures in others. Now researchers experimenting with chains of metallic beads have shown a surprising link to the physics of polymers—long, chain-like molecules that form plastic and rubber materials. Both show a marked tendency to grow stiffer in response to any deforming force. The finding could help in the engineering of stronger materials.
Push the tip of a pencil into a chunk of rubber, and the material’s resistance increases as the probe goes deeper. Researchers understand that this stiffness amplification arises because the polymers in the material are linked together. The material acts as a coherent whole, and the probe can only penetrate further by breaking contacts at many points where polymers link together.
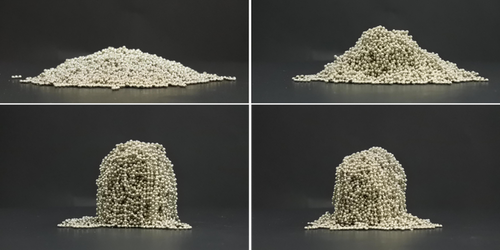
A similar linking together of separate strands occurs in the coiled rope or cable used to lift an anchor on a ship, says Pascal Damman of the University of Mons in Belgium. The more you increase tension in the rope—which is typically wrapped around a drum known as a capstan—the more the strands bind together, making them further resistant to movement. Damman and his colleagues wondered if this behavior, called self-amplified friction, might be observed for any collection of chain-like objects. They sought to test the idea in experiments using chains of metallic beads like those found in key chains or connected to sink stoppers.
They placed a collection of such chains into a cylindrical container and subjected it to vibration to allow the chains to pack themselves efficiently. They then measured the force needed to push a vertical, rod-shaped probe slowly down into the pile, recording how the force changed as the probe went deeper. The force required to deform this material is strongly influenced by the same interstrand-binding effects that cause self-amplified friction. Experimenting with chain lengths ranging from 2 to 50 beads, the team found a universal behavior in which the force always increases exponentially with the depth of the tip. They also found that the exponent describing this stiffening grows in proportion to the square root of the chain length.
This behavior, the researchers discovered, can be explained quite accurately using a mathematical model taken directly from polymer physics. This model accounts for the increase in the number of likely contacts between different chains as the chain density and length increase. The data from the experiments closely follow the pattern predicted by the model.
This agreement is somewhat surprising, the team says in their paper, given the huge differences between the behaviors of macroscopic and microscopic chains. Strongly influenced by molecular jiggling linked to temperature, polymers naturally relax to a state of thermal equilibrium, in which their shapes fluctuate over time. In contrast, temperature plays no role at all for the metallic chains, which stay locked in place, far from equilibrium.
“The agreement is remarkable,” says materials physicist Pedro Reis of the Swiss Federal Institute of Technology in Lausanne (EPFL). “The theoretical framework for polymer physics was developed for microscopic thermal systems in equilibrium.”
The results, Damman suggests, add support to a conjecture first made in 1989 by the late statistical physicist Sam Edwards, of the University of Cambridge, who believed that many properties of granular systems should be similar to those of molecular systems, even though temperature is not relevant for the granular ones. Damman says that researchers continue to debate this idea and test its validity.
The work may also have practical uses in adjusting the physical properties of common materials, Damman suggests. Self-amplified friction, he says, “appears as a kind of universal mechanism that can be used to improve stiffness in real systems.”For example, manufacturers often need to handle granular materials, such as sand, cement, flour, or pharmaceutical powders. Piles of these materials typically collapse under their own weight, but it might be possible to make them easier to control by adding in some chain-like structures, lending stiffness to the material.
This research is published in Physical Review Letters.
–Mark Buchanan
Mark Buchanan is a freelance science writer who splits his time between Abergavenny, UK, and Notre Dame de Courson, France.