Glue or Ink Improves Soft Metal Cuts
Cutting soft metals such as copper, aluminum, and some steels with precision tools is a messy affair, so these materials are often called “gummy.” But the uneven surface generated by machining can be avoided if the metal is first coated with common inks, according to recent experiments. Now the experimenters have figured out why this trick works—the coating causes the metal’s surface to become more brittle, so that it responds to the cutting tool by cracking, rather than by folding smoothly like a mushy paste. The authors say that the finding could improve cutting precision for gummy metals, which are used in many industries.
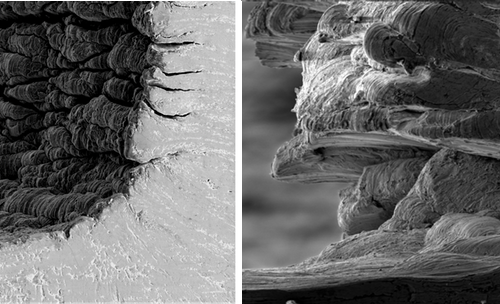
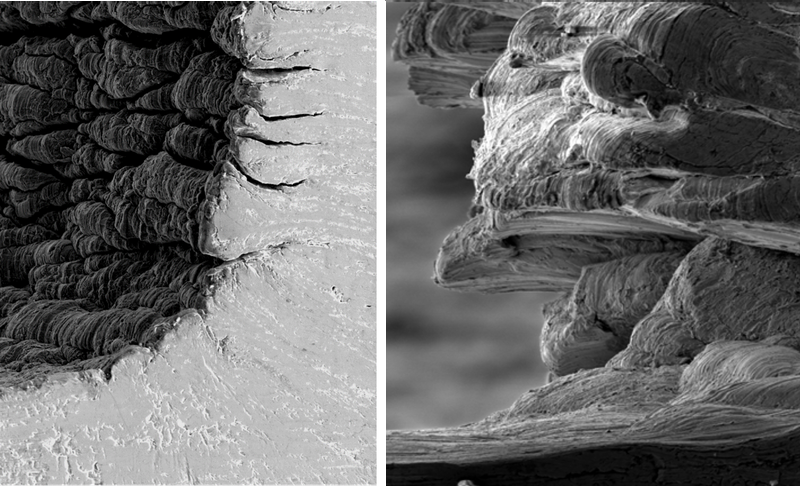
Gummy metals are used in components for robotic welders, medical implants, and many other products, but turning, milling, and drilling these materials can be costly and time-consuming, thanks to the difficulty of making clean cuts. In 2015 a team at Purdue University in Indiana, led by Srinivasan Chandrasekar, explained that the gumminess of these metals can be understood by observing the thin layer of material shaved off by a cutting blade. In a gummy metal, this “chip” forms a series of folds—what the researchers call a “sinuous” shape—as it peels away from the surface [1]. The folding may cause the chip to pull off other parts of the surface as the blade advances, leaving it pitted and rough. This behavior, paradoxically, means that a relatively large force is required to make cuts, despite the metal’s softness.
The researchers found in 2015 that the cut was smoother and required less force if they coated the metal with Dykem ink, which is commonly used to mark metals and which consists of pigments dissolved in alcohol [1]. It appeared that the ink provided these benefits by suppressing the sinuous folding. “We were as puzzled as anyone else” about why no one had noticed this trick before, says Purdue team member Anirudh Udupa.
To learn which coating properties matter most, Chandrasekar and colleagues have now systematically investigated the cutting of iron, aluminum, and copper coated with other materials, such as Sharpie permanent marker ink, glues, Scotch tape, and soap. They have also examined the cutting process under a microscope to understand why coating the metal improves the cutting process at all.
The coatings fall into three classes, they find. The first, including glues and inks, adhere strongly to the metals through physical adsorption (the presence of attractive van der Waals interactions, not chemical bonds). All such substances improve the cutting performance, producing a smoother surface and allowing cuts with less force. The second group, pure alcohols, work only for aluminum, with which they form chemical bonds. The third group, including acetone, paraffin wax, and water, has no real propensity to adsorb to the metal surface and exerts no influence on cutting.
In the presence of a strongly interacting coating, the shape of the chip is quite different from that for the bare metal. Instead of a sinuous, folded surface, the chip fragments repeatedly into slab-like segments 0.1-0.2 mm thick. In other words, the metal deforms in a brittle, rather than ductile, fashion.
The team applied a simple model of metal deformation to learn what causes this ductile-to-brittle transition. Brittle materials can crack, while in a ductile material cracks can be suppressed. Instead of advancing, a crack can dissipate its energy by radiating dislocations—shifts of one layer of the crystal with respect to the next that can propagate through a crystal. By lowering the metal’s surface energy, a coating makes it harder for dislocations to form and spread and thus undermine cracks, so the metal fractures in a brittle way.
“Our initial hypothesis was that there was a particular chemical in all of these media that was inducing the transition,” says Udupa. “But we soon realized that the effect was due simply to the fact that the media stick to the surface.”
The researchers say that the “mechanochemical” effect they identify could improve the quality and lower the cost of machining and surface finishing of these metals. For example, the right coating could improve engineers’ ability to machine gummy, nickel-based alloys, which are used for corrosion-resistant equipment in oil and gas prospecting.
This research is published in Physical Review Applied.
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- H. Yeung, K. Viswanathan, W. Compton, and S. Chandrasekar, “Sinuous Flow in Metals,” Proc. Natl. Acad. Sci. U.S.A. 112, 9828 (2015).