Watching Wood Dry
An understanding of the wood drying process at a microscopic level could help sellers of lumber and firewood dry their products more efficiently. However, there is no complete theory for the drying of this complex material. New experiments using MRI and x-ray imaging reveal that water deep in wood pores must soak into the solid material and then diffuse to the surface in order to escape, rather than finding routes to the surface through connected voids [1]. The results may have relevance for the drying of similar materials in industrial settings, such as food products.
It takes more energy to burn a wet log than a dry one, so commercial firewood needs to be dry, as does construction lumber, to prevent warping. Many researchers studying the drying process believe that it occurs in two stages. In the first, liquid water evaporates at the surface, creating capillary forces that draw internal water outward. In hardwoods, this “free water” flows through interconnected voids (softwoods lack these large pores). In the second stage of drying, the picture is less clear, but researchers call it “diffusion dominated.” This term can refer to water vapor escaping from voids or to “bound water”—which has soaked into the solid portions of the material—diffusing to the surface, or both. Many researchers also believe that during this stage, a dry layer forms at the surface and grows in thickness, says Philippe Coussot of Gustave Eiffel University in France.
However, the physical details of the two stages and the transition between them—especially regarding their relationship with the unusual microscopic structure of wood—have not been well understood, Coussot says. And the few experimental studies have given some conflicting results. To investigate the problem at both macroscopic and microscopic scales, Coussot and his colleagues used several techniques that had not previously been combined for this purpose, including MRI and x-ray imaging.
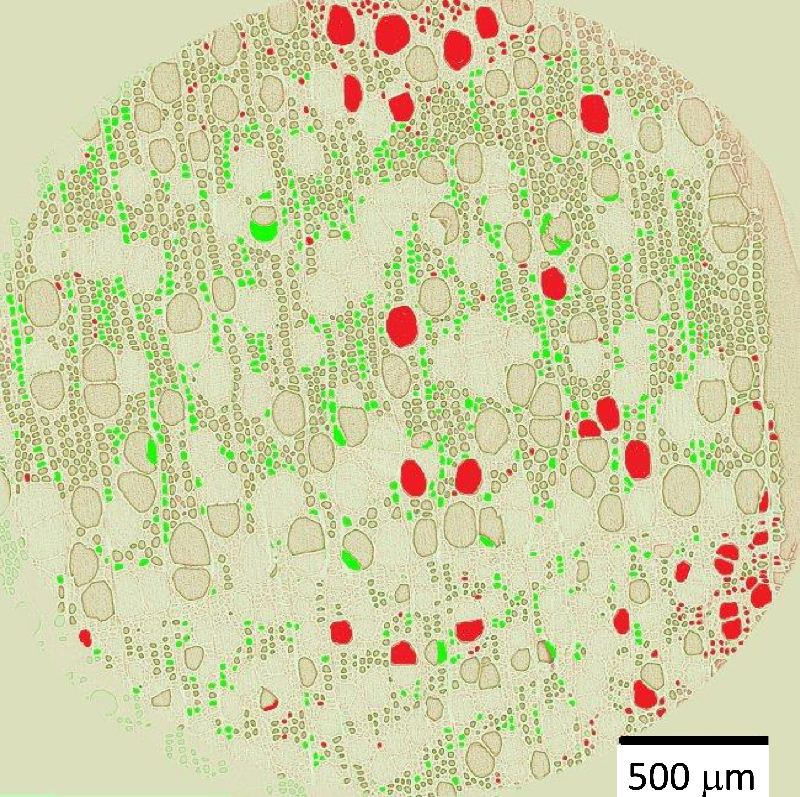
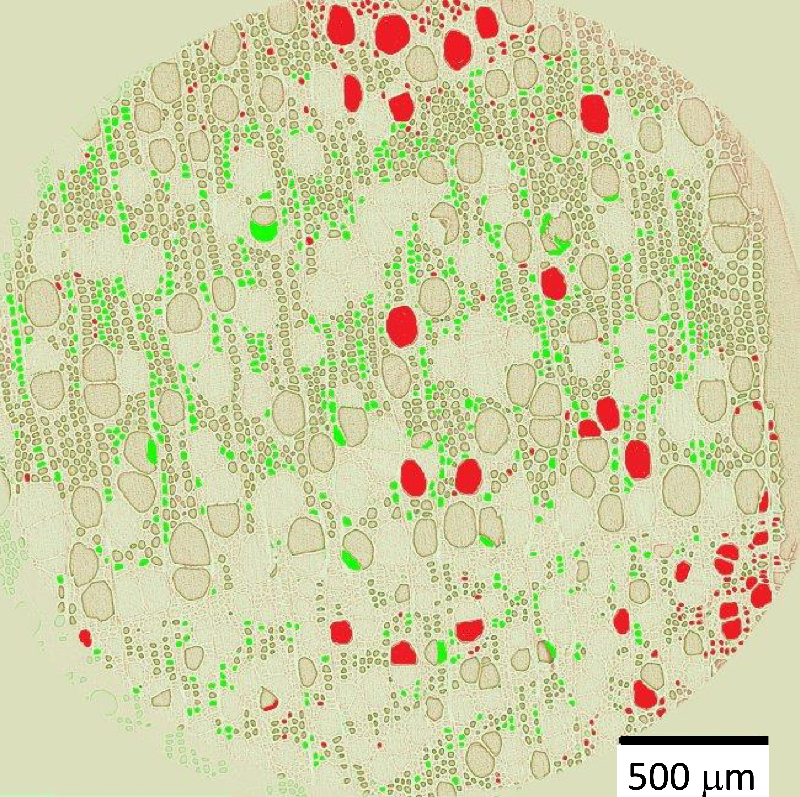
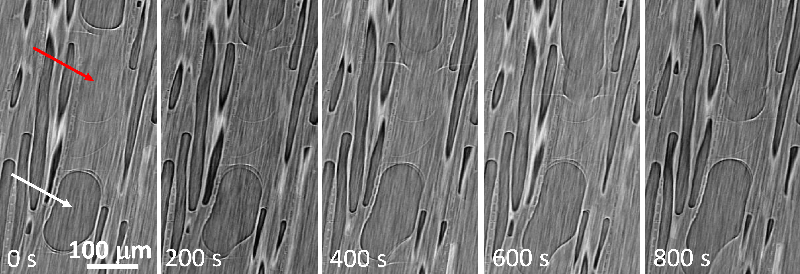
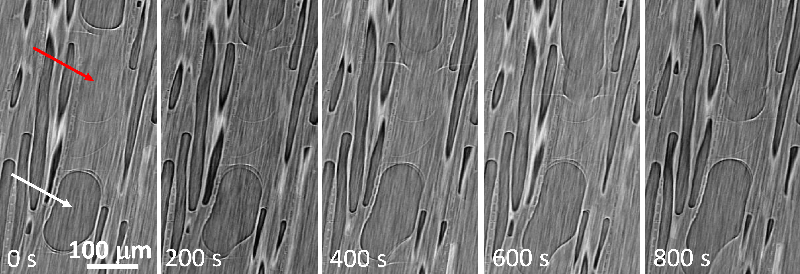
The team observed the drying of cylindrical samples of freshly cut poplar (a hardwood) or poplar left soaking for a few days, often measuring for over a week. Each sample was between 0.5 and 4 cm thick in all three dimensions, and all faces but one were covered with impermeable tape, to limit evaporation to a single surface. With the exposed surface at the top, the water conduits were oriented vertically. In hardwoods, these pores come in two types: vessels, which are long, 100-micrometer-wide tubes; and fibers, which are short, 10-micrometer-wide cavities. During drying, the researchers used nuclear magnetic resonance (NMR) to track the amount of each population of water remaining in the sample—bound water, free water in fibers, and free water in vessels.
With a relatively slow initial drying rate (controlled in the lab by airflow rate, for example), the team found that for most of the process, the drying rate remained constant. This observation suggested that a sufficient amount of water must have been continuously transported to the open surface to keep the surface wet. Such water transport has been seen in simple porous systems, such as tight packings of beads, but in those cases, the fluid flows through a continuously connected network of pores and is extracted by capillary action. Wood lacks a continuous network, at least for the short fibers, so the team reasoned that bound water must be involved.
The NMR data showed that bound water started disappearing only when all of the free water had left, and x-ray computed tomography (XRCT) images revealed droplets of free water in vessels apparently being absorbed into the walls. Finally, the MRI showed that the free water remained distributed evenly throughout the sample as it gradually disappeared, suggesting that whatever was pulling the water up was effective all the way to the bottom of the sample.
Coussot and his colleagues concluded that free water doesn’t simply flow up through vessels via capillary action, nor does water vapor diffuse through pores in a significant way. Instead, the evaporation at the surface is mainly from bound water that continually diffuses upward, which causes free water to be sucked into the walls of vessels and fibers throughout the sample to become part of this “flow” of bound water. After the free water is gone, bound water continues to diffuse to the open surface. There is also no expanding dry layer, as is commonly assumed, at least for slow initial drying rates.
“Given how ubiquitous this process is in applications, the authors’ measurements of the water profile in wood as it dries are important and widely useful,” says biological and chemical engineer Sujit Datta from Princeton University. “They were able to show that diffusion of bound water controls the [wood] drying process, unlike other typical porous media.”
–Ruma Arabatti
Ruma Arabatti is a Physics science writing intern.
References
- H. Penvern et al., “How bound water regulates wood drying,” Phys. Rev. Applied 14, 054051 (2020).