High-Resolution Technique Tracks Blood Flow in Live Fish
A new microscopy technique allows high-resolution, 3D tracking of tiny particles, enabling researchers to produce movies of fluid motion in living tissue. The system determines the locations of small particles with a precision of 30 nanometers within a volume several micrometers across and enables a high density of particles to be imaged throughout the sample. Researchers used the new system to track blood flow in a live fish by imaging tracer particles, and they say that it could be further developed to image single molecules in tiny biological structures.
The resolution of an optical microscope is limited by diffraction effects, which cause blurred images of an object that is smaller than the imaging wavelength. Resolution is also limited by distortions called aberrations that result from imperfections in the imaging process. Nonetheless, by finding the center of the blurred spot—called a point-spread function (PSF)—produced by a point-like particle, objects can be localized within the imaging plane to a precision of around 10 nanometers or less using optical techniques.
However, such methods don’t work well in three dimensions because an axial (out-of-focal-plane) displacement of the object smears and distorts the PSF. One way to determine the axial position is to purposely introduce some aberration into the optics to “shape” the PSF in a way that changes predictably with axial displacement. This idea has been used for 3D imaging [1, 2], but deducing positions from these complex PSFs is complicated and becomes almost impossible if the PSFs from several objects overlap.
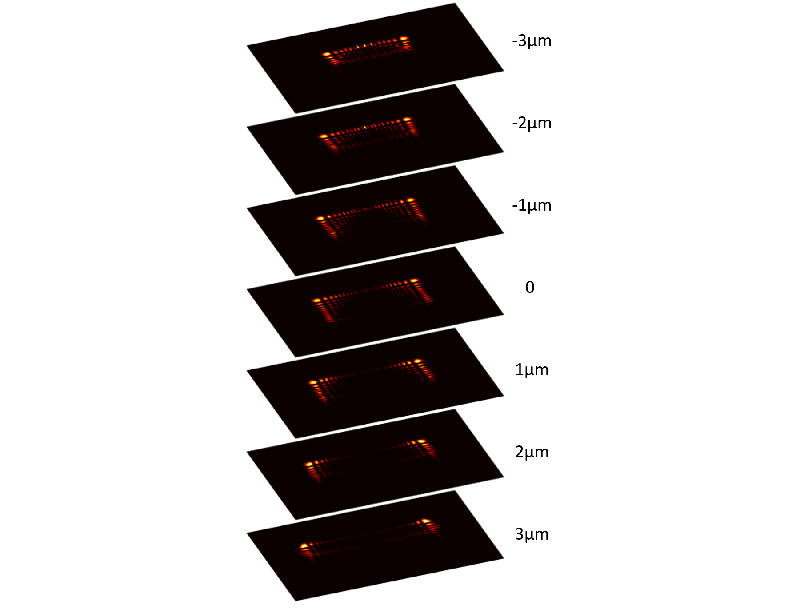
Six years ago, researchers proposed using imaging optics that modify the PSF to produce the intensity pattern of a so-called Airy beam, a type of light beam that doesn’t spread as it propagates (see Light Beam with a Curve) [3, 4]. Andy Harvey at the University of Glasgow in the UK and his co-workers have previously developed the idea, making it easier and cheaper to implement [5]. Now they have introduced an improved version of the modified PSF, called a twin Airy beam, that solves the problems that arise for multiple, closely spaced particles and uses a simpler optics setup. This new version also increases the field of view and the axial range and simplifies the calibration needed to obtain position measurements.
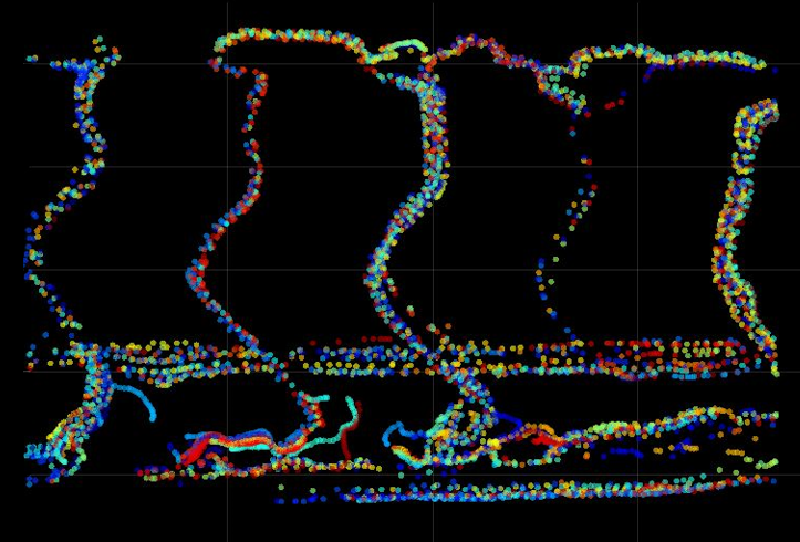
The team’s technique uses a microscope to image the fluorescence emitted by nanometer- and micrometer-sized particles in water in response to laser light. Before it reaches the detector, the fluorescent light goes through an optical device called a phase mask, a glass element laser-polished into the form needed to shape the light into a twin Airy beam. This PSF consists of a pair of bright spots surrounding each particle in the fluorescence image, along with other dimmer spots. The distance between the two bright spots increases with axial distance, and computational analysis of the pattern reveals the particle’s precise axial position.
Harvey and colleagues demonstrated a proof of principle by imaging fluorescent polymer nanocrystals, showing that the technique could locate individual particles with a precision of about 30 nanometers over an axial range of 7 micrometers. They then imaged blood flow in a zebrafish by following fluorescent polymer beads about 1 micrometer in diameter—almost 10 times smaller than red blood cells and therefore unlikely to disturb normal flow. The researchers could locate the beads to within 40 nanometers over a depth range of 0.1 mm, at a rate of about 26 frames per second, and at higher particle densities than could be resolved using other types of PSF.
“Our blood-flow measurements are not possible by other methods,” says Harvey, “since [other techniques] don’t have the combination of imaging volume, recording speed, and precision.” He says that it should be feasible to carry out local chemical sensing in living tissues by using nanoparticles that fluoresce in response to the presence of oxygen or low pH, for example. The team is now also using the new method to look at the forces exerted by growing cells, studying how the cells deform a gel medium loaded with fluorescent tracer beads.
“This is a very good piece of physics with potential for major applications in particle tracking and super-resolved imaging,” says optical physicist Kishan Dholakia of St. Andrews University in the UK. “Such systems can shed light on biological processes in ways we have not seen before.” He adds that the method could conceivably be used to image across several distance scales—for example, “to record a wide field of view and see highly resolved features at the same time.”
This research is published in Physical Review Letters.
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- Y. Shechtman et al., “Precise three-dimensional scan-free multiple-particle tracking over large axial ranges with tetrapod point spread functions,” Nano Lett. 15, 4194 (2015).
- Y. Shechtman et al., “Optimal point spread function design for 3D imaging,” Phys. Rev. Lett. 113, 133902 (2014).
- M. V. Berry and N. L. Balazs, “Nonspreading wave packets,” Am. J. Phys. 47, 264 (1979).
- S. Jia et al., “Isotropic three-dimensional super-resolution imaging with a self-bending point spread function,” Nat. Photon. 8, 302 (2014).
- Y. Zhou et al., “High-speed extended-volume blood flow measurement using engineered point-spread function,” Biomed. Opt. Express 9, 6444 (2018).