Simulations Suggest Flu Virus Vulnerability
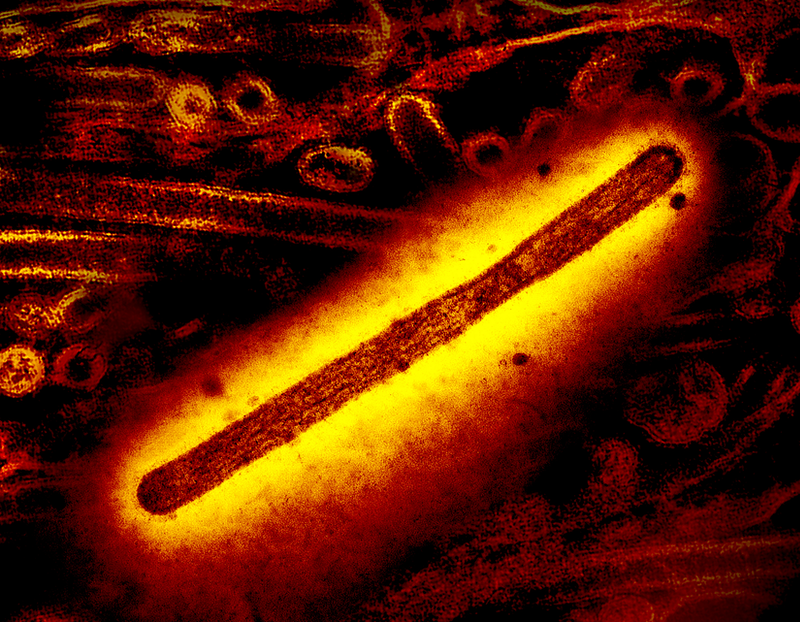
An influenza A virus (IAV) moves through the mucus that lines its host’s airways using an unusual strategy that involves binding to the mucous material to pull itself through. After simulating this process, researchers have now developed a model that predicts IAV’s speed given the properties of the proteins involved [1]. The research team identified the parameter range that enables robust forward motion and found that the protein that binds to the mucus would be a far better antiviral drug target than the other main protein involved in the locomotion.
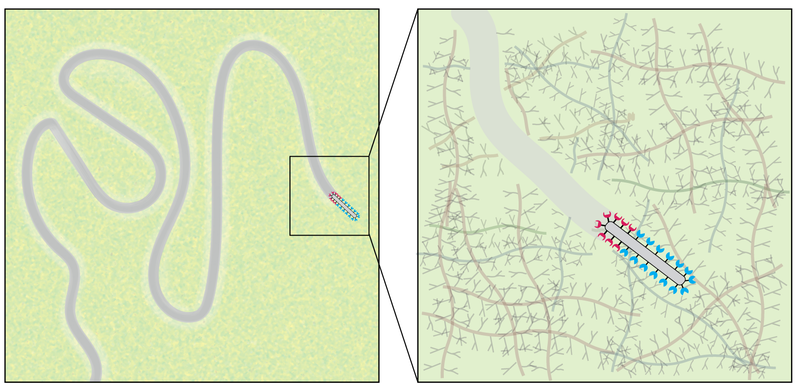
When a bacterium swims through its environment or a chromosome is moved into position during mitosis, a molecular motor powered by chemical energy is responsible. These motors produce most of the directed motion in biology. But in 2019 Mike Vahey and Daniel Fletcher of the University of California, Berkeley, discovered that IAV uses an alternative approach. It moves through the tangle of microscopic fibers comprising the mucous layer lining our airways by grabbing receptor molecules found on each fiber’s surface [2]. The grabbing is accomplished by the protein hemagglutinin (HA), which is distributed on the virus’s surface, and which continuously binds to and unbinds from the mucous receptors. At the same time, another virus protein called neuraminidase (NA) breaks off receptors, preventing HA from reconnecting after some period of time, to avoid backtracking. The whole process is called a burnt-bridge mechanism.
However, the details of this mechanism remain unclear. A complete theory for this type of viral motion is vital for understanding the onset of many infectious diseases and for developing new antiviral strategies, says Siddhansh Agarwal, a biophysicist at UC Berkeley and the research organization Chan Zuckerberg Biohub in San Francisco. To better understand the protein-binding activity involved in this viral motion, Agarwal, Fletcher, and their colleagues used simulations and theoretical modeling. They investigated the effects of variations in the HA–receptor binding affinity and the NA’s receptor cleavage rate. The bonds with receptors were represented as springs that exert forces on the virus and propel it forward.
The researchers started with a situation where the HA and NA proteins were evenly distributed across the virus surface, and they assumed the virus to be rod shaped. In this scenario, efficient movement only occurred within a narrow range of cleaving rates and binding strengths. However, when the two types of proteins were clustered at opposite ends of the virus, the movement became much more robust. This distribution stabilized the burnt-bridge mechanism and enabled faster movements across a broader range of binding affinities and cleaving rates.
Consistent with these simulations, the team developed an analytical model for IAV velocity. The model indicates that in the optimal transport regime, binding must be strong enough to provide traction but weak enough to allow movement. Moreover, the model shows that IAV locomotion is largely insensitive to NA cleaving activity but has higher sensitivity to HA binding affinity. Measured binding and cleaving rates for different IAV strains suggest that each strain evolved specific properties that optimize its transport for the mucous environment of a specific host. “Biology is wonderfully complex and messy, but finding simple physical rules that govern behavior—especially principles like the [optimization of] receptor binding strength—is deeply satisfying,” says Agarwal.
Nancy Forde, a biophysicist at Simon Fraser University in Canada, agrees. “What is particularly notable is that the parameters of human IAV align extremely well with this optimal range from their model,” she says.
Agarwal points out two important implications: First, developing drugs that target virus–receptor binding strength could be an effective therapeutic strategy, particularly if they push viruses outside the predicted Goldilocks zone. Second, understanding these binding patterns might help researchers predict and manage the risk of viruses jumping between species, since successful cross-species transmission requires maintaining efficient mucous transport in the new host.
Forde explains that while drugs currently target the receptor-cleaving protein, the new model shows that reducing its activity will not dramatically affect IAV’s ability to undergo directed motion. Instead, the researchers propose targeting the receptor-binding protein.
–Rachel Berkowitz
Rachel Berkowitz is a Corresponding Editor for Physics Magazine based in Vancouver, Canada.
References
- S. Agarwal et al., “Kinetics and optimality of influenza A virus locomotion,” Phys. Rev. Lett. 133, 248402 (2024).
- D. D. Vahey and D. A. Fletcher, “Influenza A virus surface proteins are organized to help penetrate host mucus,” eLife 8 (2019).