Easing the Squeeze on Superconductors
One of 2020’s hottest physics results was the demonstration that a hydrogen-rich material can conduct without electrical resistance at , the average temperature of a spring day in Paris. But this achievement required another condition that isn’t fulfilled on the banks of the Seine: a pressure of 267 GPa, which is close to that of Earth’s core. Researchers, including Lilia Boeri of Sapienza University of Rome, are optimistic about the prospects of easing this requirement. Boeri presented two possible pathways to achieving superconductivity at closer-to-ambient conditions at the recent March Meeting of the American Physical Society.
The road to room-temperature superconductivity traces back to a 1960s prediction that hydrogen, if sufficiently squeezed, could turn into a metal that superconducts at high temperatures. While the pressure for hydrogen metallization is exceptionally large, superconductivity hunters sought similar effects at lower pressures using hydrogen-rich compounds called hydrides. The discovery of the first hydride superconductor finally came in 2015, sparking a “hydride rush” that continues to shatter record after record for superconducting temperatures.
According to Boeri, much of this progress was spurred by theoretical advances of the last 15 years. Armed with a new density-functional theory for superconductors and with ever more powerful crystal-structure-prediction methods, theorists have guided experiments by calculating, from first principles, what potential superconductors can be synthesized and what the critical temperatures are at which their superconductivity kicks in.
These theories have guided efforts to increase the superconducting temperatures of hydrides, pinpointing a few key ingredients. For instance, highly symmetric crystal structures, high-frequency phonons, and “stiff” covalent bonds among the atoms can all boost the strength of electron-phonon coupling in the material, raising the critical temperature. Metallic hydrogen should have all of these features but only at prohibitive pressures. But in hydrides, introducing atoms into the lattice produces a “chemical” pressure that can squeeze the hydrogen lattice from within, lowering the external pressure that needs to be applied to synthesize the crystal and achieve superconductivity.
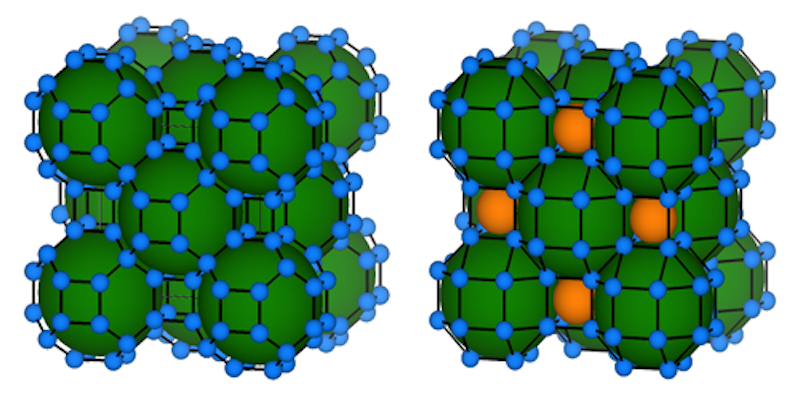
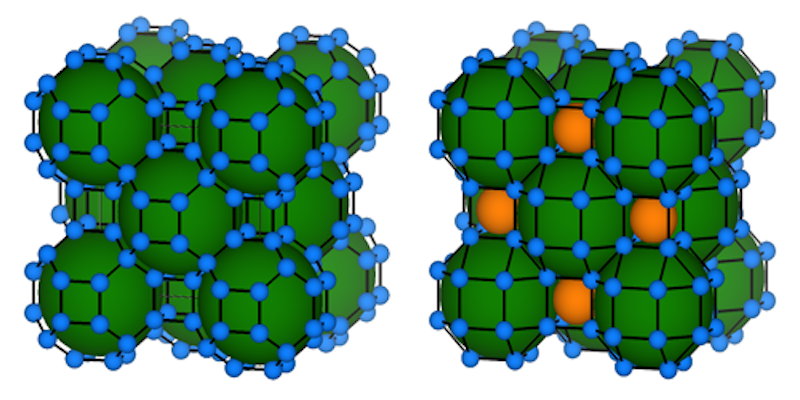
This chemical pressure can be obtained by combining hydrogen with larger atoms, and researchers have previously created hydride superconductors using elements such as lanthanum, sulfur, and yttrium. Theorists have already predicted critical temperatures for binary hydrides made by combining hydrogen with just about every other element. But none of these materials is projected to work at dramatically lower pressure, so Boeri and her collaborators came up with two new research directions.
The first one involves adding a third element to the hydride, something that increases the number of possible structures and, therefore, the options for optimizing chemical pressure. Boeri and collaborators studied a ternary hydride synthesized from lanthanum and boron. Their predictions suggest that, at 40 GPa, such a compound should both be stable and superconduct up to a temperature of 100 K.
That’s still a massive pressure, but crossing the 100-GPa threshold would be a boon to the field, Boeri says. “I could just go to the lab next door and ask a chemist to synthesize that compound for me,” while only a few facilities worldwide can do that when 100-GPa pressures are needed. That would make experiments quicker to perform, allowing for a faster exchange of ideas between theorists and experimenters, which would significantly speed up the hunt, she says.
The second direction Boeri is following involves replacing the hydrogen with other light atoms. Materials made from boron and carbon also form covalent bonds and host phonons with frequencies suitable for high-temperature superconductivity. Boeri’s team is scrutinizing a large database of boride and carbide structures with the aim of developing reliable formulas for predicting their critical temperatures. The projected temperatures are lower than those of hydrides, but these materials form crystals that are stable at ambient pressure, making them easier to work with.
This line of research is “fundamentally interesting,” says Marvin Cohen, a physicist at the University of California, Berkeley. Cohen has long believed that the secret for increasing superconducting temperatures lay in covalent bonds and says that these new compounds allow for testing of this idea. And, if the microscopic theories for these systems hold and researchers demonstrate a pressure reduction to 40 GPa, as Boeri predicts, “there is no fundamental reason why they couldn’t go all the way to ambient pressure,” he says.
–Matteo Rini
Matteo Rini is the Editor of Physics Magazine.