Active Particles Crystalize
Collections of small particles can imitate atoms by forming the equivalents of solids, liquids, and gases, following the rules of thermodynamics. However, those rules don’t apply to many systems in the natural world because they are not in equilibrium. Of special interest are systems, such as motile bacteria, that consume energy to power the movement of individual particles, often leading to self-organizing behavior. Now Phillip Geissler, of the University of California, Berkeley, and his colleagues, have used computer simulations to map out the full phase diagram of perhaps the simplest model of self-propelled particles and have found a surprise: a well-known state of these particles—in which regions of liquid and gas coexist—is not completely stable but eventually crystallizes into a solid if conditions are right [1].
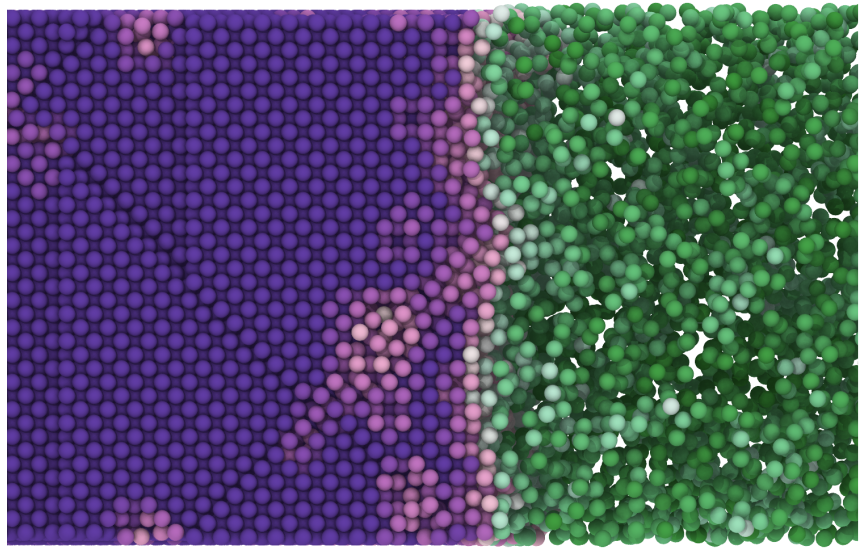
The team studied active Brownian particles (ABPs), which are spheres whose propulsion is steady but whose direction varies continuously and randomly. They looked at the behavior of the particles when varying two parameters: the strength of the propulsion and the density of particles. The resulting 2D phase diagram included a large region of liquid-gas coexistence known from previous work as motility-induced phase separation (MIPS).
In equilibrium systems, a liquid-gas coexistence phase (the equivalent of the MIPS state) can crystallize if conditions shift, but it hasn’t been clear whether a solid phase exists near the MIPS state in the phase diagram for nonequilibrium systems. Geissler and his colleagues found that nearly all of the MIPS region of the phase diagram was metastable—not stable for long times—and that, in fact, the particles in this state eventually transition to a coexistence of solid and liquid or solid and gas. Since a preference for solid-gas coexistence is already known for most equilibrium systems, the new observations suggest that the behavior of both types of particles might ultimately be explained with some kind of unified theory, according to Berkeley team member Ahmad Omar.
–David Ehrenstein
David Ehrenstein is a Senior Editor for Physics Magazine.
References
- A. K. Omar et al., “Phase diagram of active Brownian spheres: Crystallization and the metastability of motility-induced phase separation,” Phys. Rev. Lett. 126, 188002 (2021).