Probing the Limits of Nuclear Existence
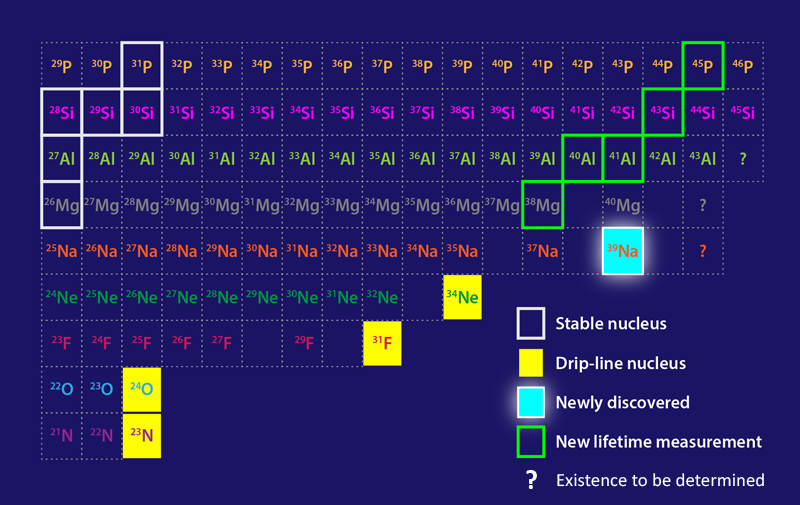
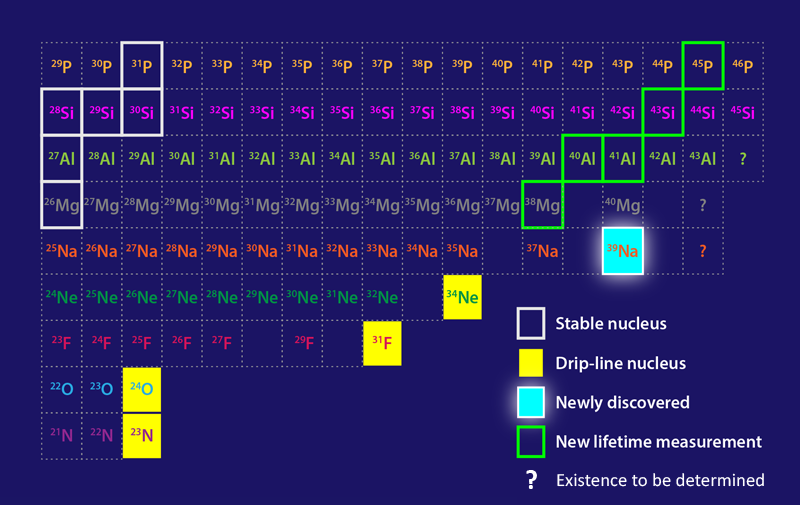
The neutron dripline marks a boundary of nuclear existence—indicating isotopes of a given element with a maximum number of neutrons. Adding a neutron to a dripline isotope will cause the isotope to become unbound and release one or more of its neutrons. Mapping the dripline is a major goal of modern nuclear physics, as this boundary is a testing ground for nuclear models and has implications for our understanding of neutron stars and of the synthesis of elements in stellar explosions. Now studies by two groups extend our knowledge of the properties of nuclei close to the dripline [1, 2]. Working at the Radioactive Isotope Beam Factory (RIBF) in Japan, Deuk Soon Ahn of RIKEN and colleagues have discovered sodium-39 (39Na), which likely marks the dripline location for the heaviest element to date (Fig. 1) [1]. Meanwhile, in the first experiments performed at the recently inaugurated Facility for Rare Isotope Beams (FRIB) at Michigan State University, Heather Crawford of Lawrence Berkeley National Laboratory and colleagues have studied neutron-rich isotopes of elements close to sodium, delivering measurements of previously unknown isotope lifetimes [2].
The atomic nucleus comprises neutrons and protons whose cohesion is ensured by one of the four fundamental forces: the strong nuclear force. The number of protons (Z) in the nucleus defines the chemical element. Nuclei of a given element with different numbers of neutrons (N) are called isotopes. Nuclei and their isotopes can be represented in the 2D Segrè chart, which shows all bound isotopes, including stable ones—those making up most of the world around us—and radioactive isotopes—which decay toward the stable ones. For a given element, neutrons can be added until the strong nuclear force is no longer strong enough to bind the nucleus together, causing neutrons to “drip out.” This limit on the neutron-rich side of the chart is the neutron dripline.
After more than a century of nuclear research, elements have been discovered all the way to Z = 118 (oganesson). It is thus remarkable that the neutron dripline is known only up to neon (Z = 10), where it is located at N = 24. Two challenges complicate the extension of the dripline: the tiny probability with which the most neutron-rich nuclei can be produced in the lab and—once a putative dripline isotope has been observed—the difficulty of ruling out the existence of even more neutron-rich isotopes of the same element. To produce these elusive nuclei, researchers use accelerator facilities where intense, high-energy beams of stable but already neutron-rich nuclei are smashed onto solid targets, causing nuclear reactions with target nuclei that produce a slew of different nuclides.
To produce 39Na, Ahn and colleagues start with a 16-GeV beam of 48Ca nuclei, which impinge at a rate of 3 × 1012 nuclei per second on a target of Be atoms. The nuclides emerging from the collisions then traverse a series of magnetic spectrometers tuned to maximize the transmission of 39Na nuclei and minimize that of contaminants. At the output of the spectrometers, nuclear detectors determine the Z and N values of each nucleus. The team reports nine detection events of 39Na collected over a two-day experiment in which approximately 5 × 1017 48Ca nuclei impinged on the Be target—a detection more challenging than finding the proverbial needle in a haystack!
The existence of 39Na provides information about nuclear stability and structure, offering an important benchmark for nuclear models. A remarkable feature of the dripline, which is located at N = 16 for oxygen (Z = 8), is that it extends to 22 for fluorine (Z = 9); to 24 for neon (Z = 10); and, as revealed by the new data, to at least 28 for sodium (Z = 11). This feature implies that as Z increases, an enhanced binding energy allows nuclei to incorporate more neutrons while remaining bound. Previous studies suggest that such additional binding ability is likely the consequence of the deformation of nuclei: 24O, for instance, is presumed to have a spherical shape [3], while neutron-rich nuclei with Z ≥ 9 are thought to have a prolate shape, resembling a rugby ball. This behavior can be explained within the framework of the nuclear shell model, first elaborated by Nobel laureates Maria Goeppert-Mayer [4] and Hans Jensen [5] and perfected over the past 70 years. In nuclear-shell-based models, protons and neutrons occupy different shells, and deformation is induced when the energy ordering of these shells changes, which can happen when neutrons are added.
The observation of 39Na offers an important validation for these models. In particular, a sophisticated shell model proposed in 2020 correctly predicts not only the position of the dripline for oxygen, fluorine, and neon but also the existence of 39Na [6]. Other cruder models, however, fail to explain the new finding. For instance, the finite-range droplet model [7], which treats the nucleus as a liquid drop, and the so-called Hartree-Fock-Bogoliubov mass model [8] both predict 39Na to be unbound. Note that the discovery of 39Na does not rigorously prove experimentally that 39Na sits at the dripline, since the experiments do not rule out the possibility that neutron-richer Na isotopes exist. However, the shell model that correctly predicts 39Na also indicates that it is the most neutron-rich bound isotope of Na [6].
The discovery of an isotope is but a first step in the study of nuclear structure. Knowledge of the isotope’s properties, including its half-life, mass, spin, and the features of its excited states, is necessary to better constrain nuclear models and evaluate their predictive ability. Kicked off in May of this year (see Research News: Rare Isotopes for the Choosing), FRIB—designed to be the world’s leading accelerator in terms of exotic-isotope production capabilities—will allow researchers to produce and study thousands of nuclei far from stability, some of which will have been created for the first time on Earth.
Crawford and co-workers report the first results from FRIB. Their setup also involves a 48Ca beam but is tuned to produce heavier neutron-rich isotopes of elements—with Z in the range 12–15 (Mg, Al, Si, and P)—and to measure their lifetimes. The team reports hitherto unknown lifetimes for five of those isotopes. Most of the obtained values agree well with theoretical predictions—offering another demonstration of the predictive power of modern shell-model calculations [9]. However, the researchers observe an unexpected drop in half-life for 38Mg compared with lighter Mg isotopes, a small discrepancy that calls for further refinements of shell-model calculations.
FRIB’s experiments can not yet probe sufficiently neutron-rich nuclei to reach the dripline for elements in the Z = 12–15 region. The results, however, come from FRIB’s first exploratory run, in which the facility operated at less than 1% of its target ion-beam intensity. A progressive intensity ramp-up planned for the coming months will allow the facility to access a wider set of isotopes, potentially leading to further extensions of the dripline.
With the edge of nuclear existence now known up to Z = 11 (assuming that 39Na is indeed located at the dripline), a terra incognita of neutron-rich nuclei with Z ≥ 12 remains to be explored. Some of them will be created by FRIB and other new accelerators coming online, such as the Facility for Antiproton and Ion Research in Germany. Others may forever remain out of the reach of our terrestrial laboratories. Their existence and properties will have to be inferred through nuclear models, whose predictive power will be boosted by the ever more stringent tests offered by experiments such as those discussed here.
Correction (17 November 2022): A previous version of Figure 1 placed scandium (Sc) between aluminium (Al) and phosphorus (p) when it should have been silicon (Si).
References
- D. S. Ahn et al., “Discovery of 39NA,” Phys. Rev. Lett. 129, 212502 (2022).
- H. L. Crawford et al., “Crossing N = 28 toward the neutron drip line: First measurement of half-lives at FRIB,” Phys. Rev. Lett. 129, 212501 (2022).
- C. R. Hoffman et al., “Evidence for a doubly magic 24O,” Phys. Lett. B 672, 17 (2009).
- M. Goeppert Mayer, “On closed shells in nuclei. II,” Phys. Rev. 75, 1969 (1949).
- O. Haxel et al., “On the “magic numbers” in nuclear structure,” Phys. Rev. 75, 1766 (1949).
- N. Tsunoda et al., “The impact of nuclear shape on the emergence of the neutron dripline,” Nature 587, 66 (2020).
- P. Möller et al., “New finite-range droplet mass model and equation-of-state parameters,” Phys. Rev. Lett. 108, 052501 (2012).
- S. Goriely et al., “Further explorations of Skyrme-Hartree-Fock-Bogoliubov mass formulas. XIII. The 2012 atomic mass evaluation and the symmetry coefficient,” Phys. Rev. C 88, 024308 (2013).
- S. Yoshida et al., “Systematic shell-model study of 𝛽-decay properties and Gamow-Teller strength distributions in A ≈ 40 neutron-rich nuclei,” Phys. Rev. C 97, 054321 (2018).