Metal-to-Insulator Transition Similar to Water-to-Ice
When water freezes, the ice forms first in “nuclei”—tiny seed crystals that can grow or shrink and survive only if they reach a minimum size—at least according to the textbook theory. Researchers have now shown that this understanding also applies to a more complicated phase transition in vanadium dioxide (VO2), a material whose electrical properties and crystal structure both change at its so-called metal-to-insulator phase transition [1]. The team measured the threshold size for the “seeds” that drive this transition and demonstrated a new technique for studying crystal structure transitions. The result suggests that the classical nucleation theory is valid for a range of materials that are important in areas such as catalysis, lasers, and alloy and ceramic manufacturing.
Place a bucket of purified water in a subfreezing-temperature environment, and tiny ice seeds will start forming. Many will quickly dissolve, but those that are larger than a certain threshold size will grow and eventually merge to make a single block of ice. This view of crystallization, associated with classical nucleation theory, has been well accepted for the water–ice transition. Junqiao Wu of the University of California, Berkeley, and his colleagues wanted to test whether the same nucleation phenomenon is at play in VO2 when it makes a transition from one crystalline structure to another.
VO2 is used in surface coatings, sensors, and imaging systems. Above 340 K, the material is a metal, meaning that it’s a good electrical conductor. At room temperature, it becomes an insulator (nonconductor). This metal-to-insulator transition (MIT) is accompanied by a change in the material’s crystalline structure and has some quirks that are reminiscent of the water-to-ice transition. For example, if the material is pure enough, VO2 can be “supercooled” and remain metallic at temperatures where it is normally insulating. Water can also be supercooled below its freezing point.
Researchers have previously seen hints that VO2 and similar materials nucleate phase transitions the same way that water does, but they had no direct evidence. To explore this possibility, Wu and his colleagues developed a method to place “nucleation seeds”—tiny regions that can nucleate phase transitions—within the metal and to control the nucleation process.
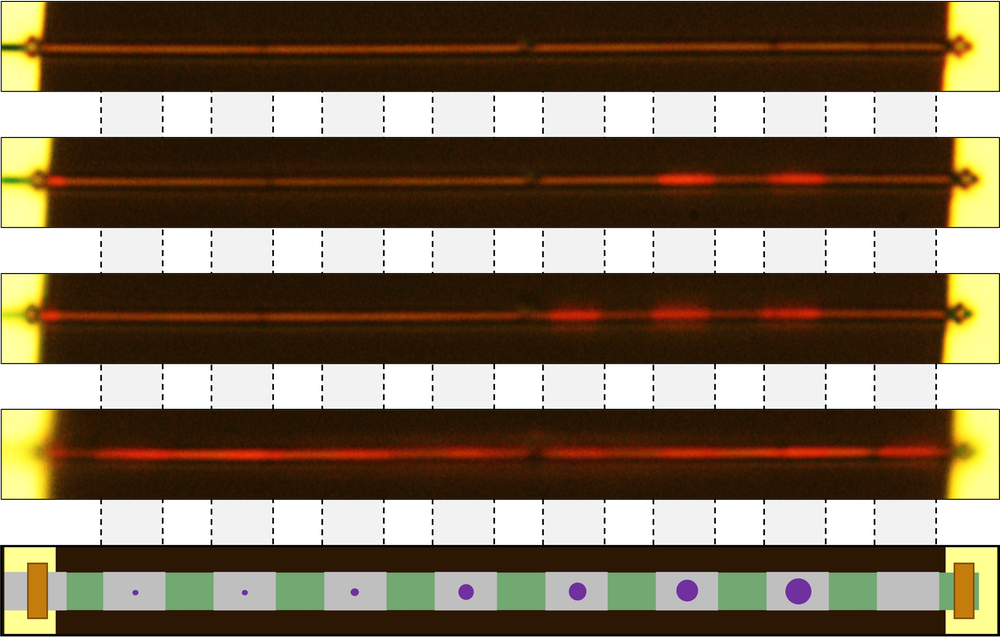
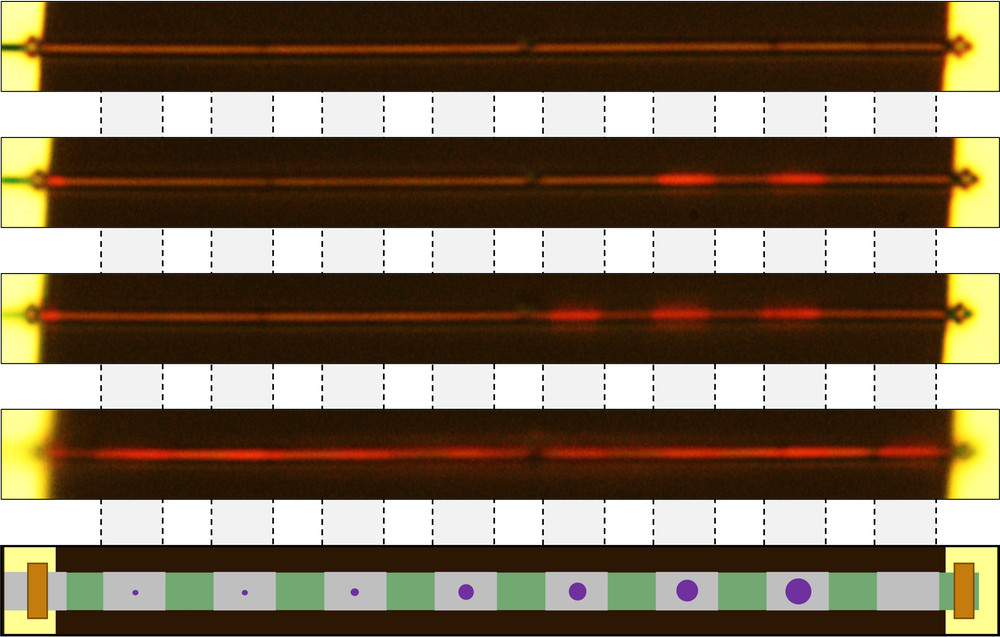
First the researchers made a series of pure, single-crystal, metallic VO2 wires with cross sections of about 100 × 250 square nanometers (nm2) and lengths of about 50 micrometers. For each experiment, they suspended one of the wires across a pair of parallel electrode ridges, so that the wire was supported only at its ends. The team then irradiated the wire’s ends with helium ions. This irradiation damaged the ends in a way that prevented contact with the electrodes from triggering unwanted nucleation, thus creating “an ideal seedbed to plant and grow [nucleation] seeds,” Wu says. They also irradiated a series of stripes across the wire to create eight separate, “shielded” segments. The team used a focused gallium ion beam to distort the crystalline structure and create a nucleation seed in each segment. The seeds ranged from 10 nm up to 180 nm in diameter, and the team monitored their effects on the wire’s phase transition with an optical microscope.
As the researchers cooled the wire below VO2’s normal phase transition temperature, each seed eventually triggered a phase transition in its segment—the smaller the seed, the lower the transition temperature. The team found that a minimum seed size of tens of nanometers was required for the MIT to take place (with the precise value depending on the details of the seed creation). All the results agreed with the predictions of classical nucleation theory.
Wu says that the results indicate that VO2’s MIT is nucleation driven and that researchers might be able to use better-studied materials to glean further insights into the MIT. “There is something universal that governs these phenomena,” he says. He also thinks that the results offer a route to designing materials with well-controlled phase transitions that could improve the use of VO2 in applications as well as in fundamental research studies. “The deeply supercooled VO2 that we demonstrated is an ideal test bed for future investigation,” Wu says.
The confirmation that classical nucleation theory is valid for the crystal-to-crystal transition in VO2 is both “important” and “exciting,” says Volker Eyert, a materials scientist at Materials Design, Inc., in France. The results demystify this specific phase transition and suggest that standard theories may apply to phase transitions in other materials, he adds.
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- L. Jin et al., “Probing the critical nucleus size in the metal-insulator phase transition of VO2,” Phys. Rev. Lett. 129, 245701 (2022).