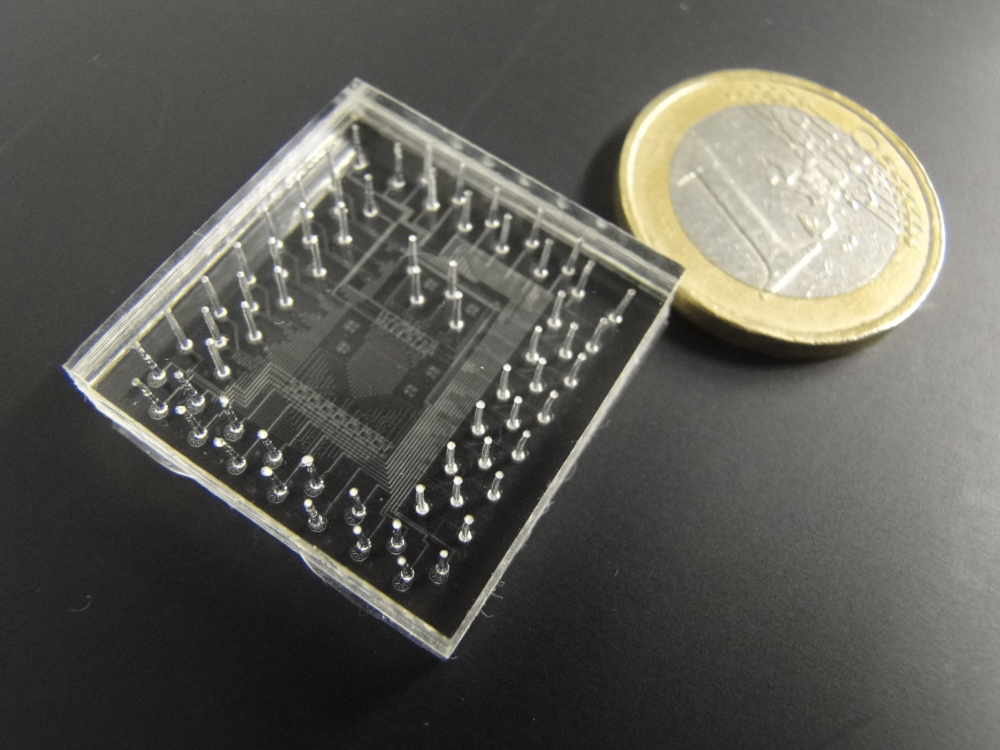
Optical Sensor for a Pancreas-on-a-Chip
An organ-on-a-chip is like a mini laboratory where drugs and other chemicals can be tested on a collection of living cells. These compact devices, which replicate basic organ functions by combining cell cultures, microfluidics, and electronics, could reduce the need for animal testing. New work on a pancreas-on-a-chip demonstrates an optical detection method that can provide real-time monitoring of the amount of insulin produced by the pancreas.
“This is the first time that optical chips are used to do real-time detection in an organ-on-a-chip,” says one of the developers, Romain Quidant, who recently moved from the Institute of Photonic Sciences in Barcelona to the Swiss Federal Institute of Technology (ETH) in Zurich, “We can actually follow the production of insulin by pancreas cells as we change the environment on the chip.”
For over a decade, researchers have been developing organs-on-a-chip that use cultured cells from the liver, brain, heart, and other organs. The cells are integrated within a microfluidic device that pumps in nutrients and evacuates wastes. Previous studies have shown that organs-on-a-chip can reproduce biological functions, such as the response of lung tissue to bacterial infections. One of the main motivations of organ-on-a-chip technology is that it could replace animal testing. “You can reproduce or mimic the functionality of specific tissues, and then you can use the chips to test drugs or study diseases,” explains Javier Ramón-Azcón from the Barcelona Institute of Science and Technology in Spain.
But the monitoring of organs-on-a-chip is typically done off the chip and with a significant time lag. The traditional method involves a researcher extracting fluid from the chip and then introducing antibodies that target specific molecules. When an antibody binds to its target, it produces an optical signal, such as fluorescent emission. This so-called immunoassay can take several hours to perform. Quidant, Ramón-Azcón, and their colleagues have come up with a more direct detection method that can provide real-time data. Such continuous monitoring is desirable for studying insulin production and other metabolic processes that can vary over timescales of just a few minutes. Quidant presented this work last month at the Photoptics 2022 online meeting.
The researchers’ detection method relies on the interaction between light and nanoscale objects. “With nano-optics you can do things that you cannot do with conventional optics,” Quidant says. When light strikes a nanosized sphere or rod, electromagnetic fields become concentrated on the object’s surface. Quidant’s group uses that concentrated light in a number of applications, such as photocatalysis and localized heating. For monitoring an organ-on-a-chip, they use the concentrated light to sense the presence of a particular molecule—in this case insulin.
The team’s detector consists of arrays of gold nanorods on a glass substrate. The nanorods are optical resonators that scatter light at a specific wavelength of around 800 nm. But this resonance wavelength depends on a nanorod’s surface environment. Using chemical methods, the team attaches insulin antibodies to the nanorods. Whenever an insulin molecule binds to an antibody, it induces a shift in the nanorod resonance wavelength. Quidant compares it to placing a finger on a guitar string. The antibody alters the index of refraction at the surface, exactly where the nanorod concentrates the light field. “If you introduce a tiny perturbation where the field is actually concentrated, you can have a very strong effect,” Quidant says.
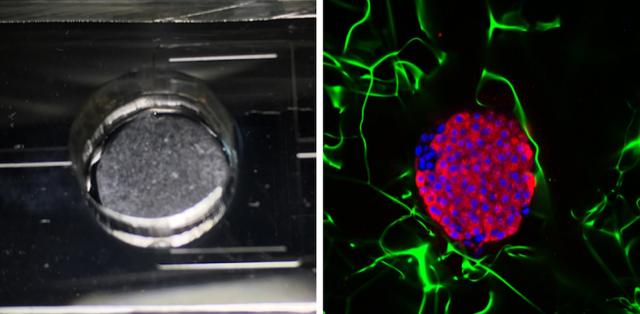
The researchers demonstrated this optical sensor by connecting it to a pancreas-on-a-chip developed by Ramón-Azcón’s biology group. For this test, the group extracted pancreas cells from mice and cultured them in 10-mm-wide porous cylinders made of cellulose. Two of these cylinders—each containing about half a million cells—were placed in a microfluidic system, through which a nutrient-filled fluid flowed at a rate of 50𝜇L (roughly a teardrop) per minute. The researchers diverted some of this fluid into a separate chip where the optical sensor was located.
To track the nanorod resonance, the team shined light on the arrays and analyzed the transmitted light with a spectrometer. By measuring tiny shifts of about 10 pm in the resonance wavelength, the team found that they could detect insulin at the level of 0.85𝜇g∕mL—a sensitivity comparable to that of conventional immunoassays. In separate experiments, the team added sugar (glucose) to the pancreas-on-a-chip and observed a rise in insulin levels, implying that the pancreas cells were functioning properly.
Building on this successful demonstration, the researchers are working in multiple directions to improve the system. Quidant’s team is trying to integrate the optical sensor directly with the organ so that the whole system can be housed in a single device the size of a smartphone. Ramón-Azcón’s team is now combining three organs—pancreas, liver, and skeletal muscle—on a single chip. This multiorgan approach will allow them to mimic organ interactions that are thought to play a role in the origin of diabetes.
Ramón-Azcón says that pharmaceutical companies have begun investing in organ-on-a-chip technology as a potential way to do high-throughput testing of candidate drugs. The small size and specific nature of organs-on-a-chip could reduce the amount of time needed for bringing promising drugs to clinical trial. “We can have more accurate predictions of human response with a chip because we are using human cells as opposed to animals,” Ramón-Azcón says.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.