Wiggling of Silver Atoms Provides Key to Thermoelectricity
Materials that convert heat into electricity or electricity into heat are predicted to be efficient energy harvesters, giving them the potential to transform green technologies. While these “thermoelectric” materials have made their way to Mars, where they help power rovers, they are rarely used on Earth, in part because of a scarcity of materials with the right mix of properties—high electric conductivity coupled with low thermal conductivity. Now, Mercouri Kanatzidis of Northwestern University, Illinois, and colleagues have identified a new material with these properties [1]. The team believes that the finding could help researchers find other thermoelectric materials.
Kanatzidis and postdoctoral scholar Hongyao Xie were studying the thermal properties of the semiconducting material silver gallium telluride ( AgGaTe2), when they noticed something unusual about its behavior. Instead of exhibiting high thermal conductivity, like its copper-based counterpart, the material appeared to have an extremely low conductivity that contradicted predictions.
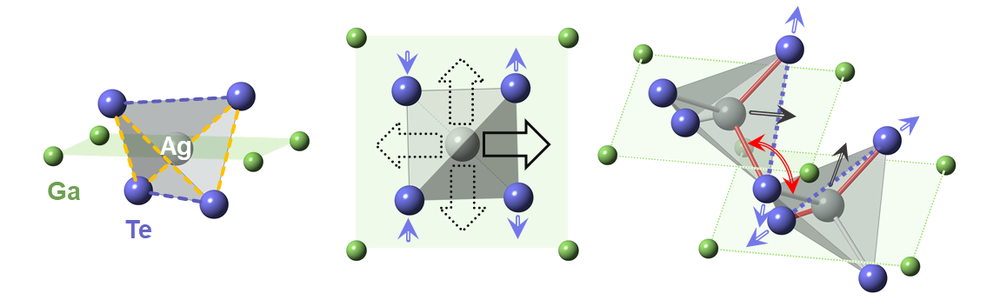
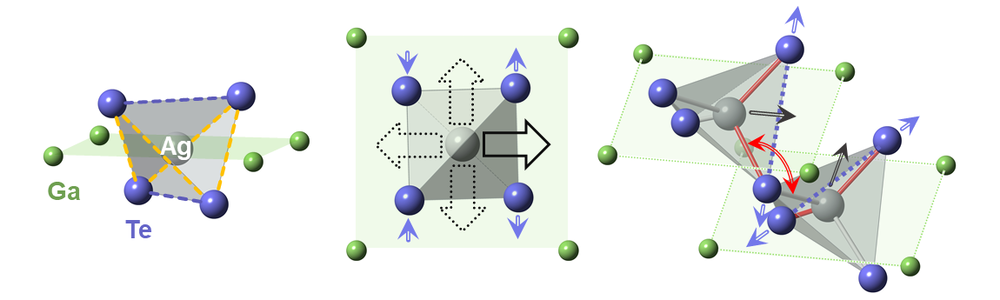
To understand the cause of this discrepancy, the duo decided to take a closer look and inspect the material’s atomic crystal symmetry. They teamed up with researchers at Brookhaven National Laboratory (BNL), New York, and utilized that facility’s synchrotron light source to study materials at the nanoscale. Observing the lattice structure of their AgGaTe2 samples, the team found that its expected diamondoid lattice—one where the arrangement of the atoms has the same spatial pattern as those in a diamond crystal—was distorted. The silver atoms did not sit where they were supposed to.
In AgGaTe2, silver atoms are expected to sit at the center of a tellurium tetrahedron, creating the diamondoid lattice structure. But the x-ray analysis revealed instead that the silver atoms’ positions were off center and that their exact locations fluctuated over time. As the team heated the material, they found that these fluctuations increased in magnitude, disrupting the lattice structure’s global symmetry. From the experimental data and from computational modeling, the team determined that this temperature-induced lattice distortion—a rare phenomenon known as emphanisis—was the root cause of the material’s low thermal conductivity, says Emil Bozin of BNL, who led the structural analysis part of the project.
“For some time, other researchers in the semiconductor community have been inspired to determine the potential of diamondoid compounds as thermoelectric materials,” Kanatzidis says. “We suspected that there was more science here than met the eye.”
The work is a “beautiful” deep dive into an area of research that could have wide ranging implications, says Zhiting Tian, a mechanical and aerospace engineer at Cornell University whose research focuses on thermal transfer, conversion, and storage. “Ultralow thermal conductivity is always favorable for thermoelectric applications, as it helps to maintain the temperature gradients between the hot and cold sides [of a device] and reduce heat loss,” she adds. “This work offers new insights into engineering diamond-type structures that possess ultralow thermal conductivity.”
The team now plans to investigate whether adding silver to other materials can induce in them similar temperature-dependent lattice deformations and thermoelectric behaviors. The next step of this research is to use this finding to see how it impacts the thermoelectric performance of other diamondoid compounds, Xie says. Beyond thermoelectricity, the team also thinks that the heat-dependent behavior of AgGaTe2 could potentially impact both how quickly electrons can move through the material under different conditions and how the material emits electrons when illuminated with light, properties important for use of the material in solar cells and other optics applications.
–Sarah Wells
Sarah Wells is an independent science journalist based outside of Washington, DC.
References
- H. Xie et al., “Hidden local symmetry breaking in silver diamondoid compounds is root cause of ultralow thermal conductivity,” Adv. Mater. 2202255 (2022).