Martian Rocks Produced Bio-Friendly Gas Long Ago
Was there ever life on Mars? The answer has eluded researchers, but several missions to our nearest neighbor have uncovered evidence that past conditions on Mars were warmer and wetter—and therefore friendlier to life. A new study of a rare type of Earth rock indicates that ancient Mars was also host to important prelife chemistry. Ben Tutolo of the University of Calgary in Canada and Nicholas Tosca of the University of Cambridge in the UK demonstrate a Martian pathway for generating molecular hydrogen ( H2). This molecule is a source of chemical energy for simple life forms and necessary for creating an environment warm enough to promote habitability and sustain liquid water.
Geochemists have long connected H2 production to olivine, a mineral that is common in Earth’s mantle. Olivine contains iron that oxidizes when water infiltrates into the rock, releasing H2. This aqueous-alteration process, known as serpentinization, leaves behind light-green, snake-skin-patterned “serpentine” rocks that provide a record of past liquid water and H2 release. Orbiter, rover, and meteorite samples indicate that Mars, too, has serpentine and olivine—albeit ones with very different compositions from their terrestrial counterparts—prompting hypotheses about if and how the same process played out during the red planet’s watery past.
Earth’s mantle is almost uniformly comprised of iron-poor, magnesium-rich olivine. The opposite is true on Mars, where iron-rich, magnesium-poor olivine is abundant. Serpentinization of iron-rich olivine should produce H2—perhaps even more than that of iron-poor olivine—but there’s been very little research to support this hypothesis. “Our limited understanding of serpentinization on Mars stems from the simple observation that the iron-rich olivine so common on Mars is in fact extraordinarily rare on Earth,” Tutolo says.
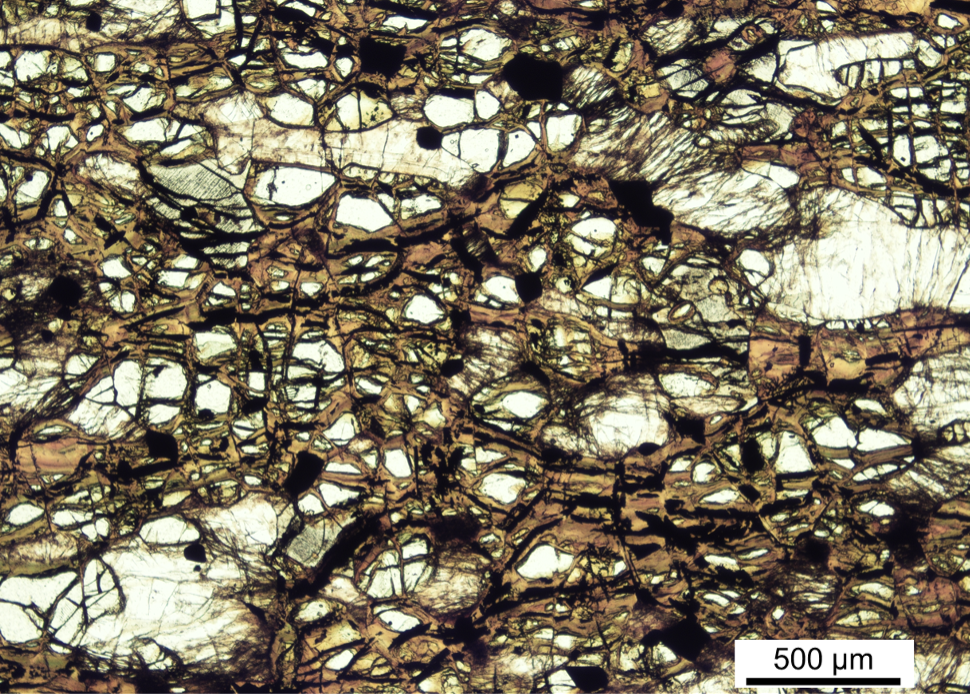
To learn how much H2 gas Mars olivine might produce, Tutolo and Tosca looked to a unique geological site in Minnesota called the Duluth Complex. There, the serpentine rocks are notable for their abundant oxidized-iron content. By examining the Duluth Complex rocks, the researchers could draw conclusions about how iron-rich rocks change during serpentinization.
The researchers analyzed Duluth Complex samples using chemical testing as well as electron and x-ray spectroscopy. Their modeling of the data—which they presented last month at the European Geosciences Union General Assembly—provides a historical account of the rocks’ evolving mineral compositions [1]. The analysis showed that—paralleling a trend that mimicked typical mantle rocks—the Duluth rocks increased in oxidized iron as serpentinization progressed. Strikingly, the iron-rich versions produced around 5 times more H2 than their magnesium-rich counterparts at any given point during serpentinization. “The results yield new constraints for models of Mars’ planetary evolution,” says Tutolo.
On Mars, serpentinization would have occurred at lower temperatures than on Earth, implying that the process would have been slower and less complete. But even weakly (20%) serpentinized rocks on Mars would have produced as much H2 as fully serpentinized rocks on Earth.
The overall rate of H2 production on Mars has important implications for the planet’s environmental conditions. Models of atmospheric evolution rely on H2 as a key greenhouse gas for raising surface temperatures on early Mars above the freezing temperature of water. Moreover, H2 is highly reactive with carbon-containing inorganic compounds such as carbon dioxide or methane. If such reactions occurred on Mars, they could have supplied organic molecules as well as energy to life forms that possibly inhabited the planet long ago.
Bethany Ehlmann, a planetary scientist at California Institute of Technology, who was not involved in the study, says “the work reinforces the fact that rocks matter when considering the evolution of a planet’s atmosphere and habitats.” Coupled with other greenhouse gases, such as carbon dioxide and methane, the geologically produced H2 could have provided the vital temperature boost required to stabilize liquid water and promote habitability on early Mars. “The key to warming Mars and generating habitats may be in environments where water interacts with volcanic rock,” says Ehlmann.
–Rachel Berkowitz
Rachel Berkowitz is a Corresponding Editor for Physics Magazine based in Vancouver, Canada.
References
- B. Tutolo and N. Tosca, “Terrestrial constraints on H2 generation during Martian serpentinization,” EGU General Assembly 2022, Vienna, EGU22-3157 (2022).