Cold Collisions Get Charged
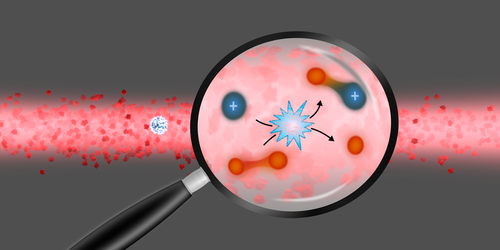
To understand the influence of quantum effects on a chemical reaction, scientists typically perform the reaction at ultracold temperatures, where they can more easily model the quantum collisions that produce molecules. Those collisions have been studied experimentally at ultralow temperatures for two atoms but not for the more complex scenario of an ion and a molecule. Now, Henrik Hirzler at the University of Amsterdam and his colleagues have changed that, observing ultracold reactions between ytterbium ions and lithium dimers [1]. The demonstration expands the kinds of reactions for which scientists can probe the quantum effects.
To make their molecules, the team took the following steps: First, they trapped a single ytterbium ion ( Yb+). Second, they prepared a cloud of ultracold lithium atoms and dimers. Third, they spatially overlapped the cloud and the ion. After letting the ion and atom cloud interact for 500 ms, they checked the Yb+ion, bathing it in resonant-frequency laser light to see if it fluoresced.
The team found that when there were more lithium dimers present in the cloud, Yb+ was more likely to have stopped fluorescing after that time period, something that indicates a shift in the spacing between the ion’s energy levels. The team showed, using mass spectrometry, that this shift was due to the Yb+interacting with a lithium dimer to form YbLi+ and a Li atom.
Now that Hirzler and his colleagues have demonstrated that they can make molecular ions, they say that they hope to study in more detail the quantum effects involved in these reactions. They think that these ultracold molecules could also be used in quantum sensors and in searches for new physics.
–Katie McCormick
Katie McCormick is a freelance science writer based in Sacramento, California.
References
- H. Hirzler et al., “Observation of chemical reactions between a trapped ion and ultracold Feshbach dimers,” Phys. Rev. Lett. 128, 103401 (2022).