Microwaves Can Suppress Chemical Reactions
According to Arrhenius’ law, heating increases the energy of molecules so that more of them can overcome the activation barrier and undergo a chemical reaction. One way to deliver heat is via microwave radiation. Since its early use in chemical synthesis, scientists have noticed that microwave-induced reactions often proceed differently compared with ones enhanced with oil baths and other traditional heating methods. This finding has led to ongoing speculation and debate—and even controversy—about the existence of microwave effects beyond heating [1]. Now Valentina Zhelyazkova of the Swiss Federal Institute of Technology (ETH) Zurich and her collaborators have demonstrated that microwaves can both speed up and slow down chemical reactions [2]. The discovery provides clear evidence of the nonthermal influence of microwaves on chemical processes. It also opens a path toward controlling reactions and understanding them more deeply.
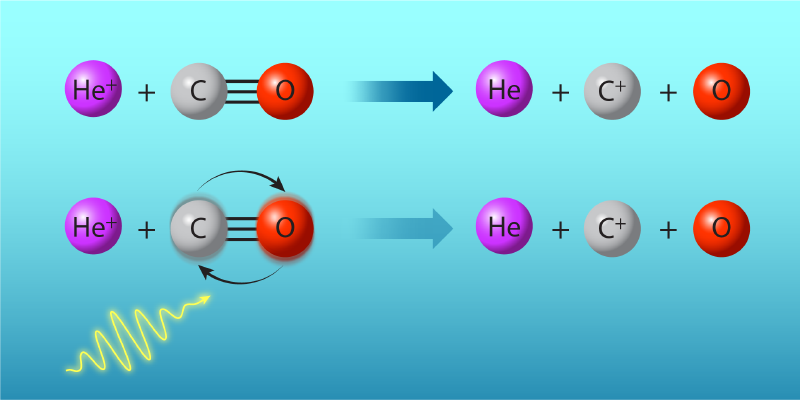
In their investigation Zhelyazkova and her collaborators manipulated the rate of the gas-phase reaction between positively charged helium ions (He+) and carbon monoxide (CO) molecules: He++ CO →He + C++ O. According to so-called capture theory, the reaction’s rate depends on the rotational states of CO, whose quantized energies lie within the microwave band (Fig. 1). The experiment began with the preparation of separate supersonic beams of He atoms and CO molecules via high-pressure expansion into vacuum. The CO molecules were initially in the rotational ground state. By applying a precisely timed microwave pulse before the reaction, the researchers excited a fraction of the population to the first rotationally excited state, which is less reactive than the ground state. The fraction that was excited could be fine-tuned by changing the duration of the microwave pulse.
Reactions occurred at collision energies corresponding to just a few kelvins, temperatures at which only a few quantized angular momentum states participate. To achieve such low effective temperatures, the researchers merged the CO and He beams into nearly parallel trajectories and carefully matched their relative velocities. The merger of the beams was facilitated by using a laser to turn the He atoms into Rydberg atoms. A device called a Rydberg-Stark deflector steered the excited He atoms into the path of the CO molecules. The conversion to Rydberg atoms had another effect. It turned helium’s electrons into distant spectators, leaving the atoms to participate in the reaction as if they were ions.
Using a time-of-flight mass spectrometer equipped with a microchannel plate detector, Zhelyazkova and her collaborators identified and counted the reaction products. They inferred reaction rates at different temperatures and compared them with capture-theory predictions. The results could not be explained by assuming that only one magnetic sublevel of rotationally excited CO was occupied, as expected from the properties of the microwave radiation. To reconcile theory with experiment, the researchers found they needed to consider the contribution of all magnetic sublevels of rotationally excited CO to the overall reaction rate. The researchers proposed that stray fields in their lab induced randomization among the sublevels. Magnetic sublevels aside, capture theory works well at low temperatures and with only one reaction pathway [3]. However, the theory does not account for short-range forces, which influence the pathway a reaction takes. Therefore, it cannot predict which products are formed and in which proportions.
Zhelyazkova and her collaborators’ microwave-control scheme not only demonstrates the ability to suppress reaction rates, but it also provides a means to finely tune chemical reactivity—from suppression to enhancement—by adjusting the microwave-pulse duration. This approach could be generalized to a wide range of molecules prepared in specific rotational states and thus allow for deeper insights into isomers—that is, molecules with identical atomic composition—or for in-depth studies of the rotational-state dependence of chemical reactions under astrophysically relevant conditions.
References
- C. Oliver Kappe, “Controlled microwave heating in modern organic synthesis,” Angew. Chem., Int. Ed. 43, 6250 (2004).
- F. B. V. Martins et al., “Microwave-controlled cold chemistry,” Phys. Rev. Lett. 134, 123401 (2025).
- A. Tsikritea et al., “Capture theory models: An overview of their development, experimental verification, and applications to ion–molecule reactions,” J. Chem. Phys. 157 (2022).