Elusive Superconducting Superhydride Synthesized
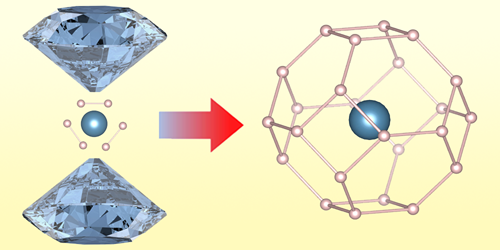
Among the various routes that physicists hope might lead to practical, room-temperature superconductors, one of the most fruitful ones involves hydrogen-rich binary compounds containing rare-earth or actinide elements. One such compound, lanthanum superhydride, has already been shown to superconduct at temperatures up to 260 K, but only under pressures greater than 170 GPa (see Viewpoint: Pushing Towards Room-Temperature Superconductivity). Now, Liang Ma and colleagues from Jilin University in China have expanded the search by synthesizing a new type of superhydride containing an alkaline-earth metal instead of a rare-earth metal or an actinide [1]. The researchers say that the synthesis of the new material, clathrate calcium hydride ( CaH6), opens the door to a class of superconductors that is currently underexplored.
The structure and superconducting properties of CaH6 were first predicted in 2012. However, subsequent attempts to synthesize the compound failed to overcome obstacles such as the high reactivity between calcium and hydrogen, which, when brought together at low pressures, can result in hydrides with low hydrogen content. In their new work, Ma and colleagues solved this issue by using ammonia borane ( BH3NH3) as a hydrogen source, which allowed them to synthesize the compound by direct reaction between calcium and hydrogen at high temperature and pressure.
In the team’s experiments, the synthesized CaH6 exhibited superconducting properties very close to theoretical predictions, achieving a critical temperature of 215 K at 172 GPa. Although this temperature is below the record set—under a similarly impractical pressure—by lanthanum superhydride, the researchers hope that experiments with other alkaline-earth-metal superhydrides will eventually lead to room-temperature superconductivity under more easily achievable conditions.
–Sarah Wells
Sarah Wells is an independent science journalist based outside of Washington, DC.
References
- L. Ma et al., “High-temperature superconducting phase in clathrate calcium hydride CaH6 up to 215 K at a pressure of 172 GPa,” Phys. Rev. Lett. 128, 167001 (2022).