Why Humidity Doesn’t Affect Drying Paint
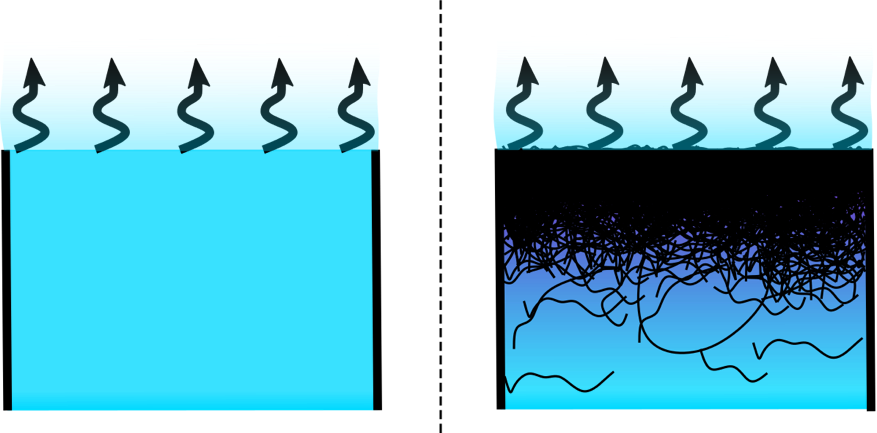
You might think that polymer solutions like paint dry more slowly on a humid day than on a dry day. But researchers have now verified a theory that explains why the evaporation rate of the water or another solvent in a polymer solution can be independent of the ambient humidity [1]. The experiments show that, as predicted, water evaporation drives the polymer molecules toward the surface, where they form a dense layer that hinders evaporation and shields the surface from humidity effects. This phenomenon may affect the rate at which virus-containing respiratory droplets evaporate and thus could help explain the seasonal dependence of viral infections.
Humidity-independent evaporation is an advantage in many situations. For example, to preserve the body’s hydration, human skin maintains a nearly constant evaporation rate thanks to cell membranes whose lipid molecules can be reconfigured to adjust the sweat evaporation rate. This reconfiguration is an example of an active process. In 2017, Jean-Baptiste Salmon, a chemical engineer at the University of Bordeaux in France, proposed that humidity-independent evaporation does not require an active response [2]. Instead, his theory suggested that it occurs whenever the solvent evaporates from a solution of large molecules, a process that was already known to draw those molecules toward the drying interface. He predicted that, after the large molecules form a dense layer, the solvent’s evaporation rate will remain unchanged whether the surroundings are bone dry or at 100% humidity. However, the theory has not been tested with a nonactive polymer solution.
“We wanted to know whether this [theory] might have implications for the evaporation of respiratory virus droplets, which also contain high-molecular-weight polymers,” says Max Huisman, a soft matter physics graduate student at the University of Edinburgh, UK. Models of viral spread neglect the influence of these biopolymers on droplet evaporation. These molecules are not expected to participate in an active process, so the first step for the Edinburgh team, led by Simon Titmuss, was to test the Salmon theory with a simple, nonactive and nonbiological polymer solution and to determine the parameter ranges over which it applies.
The researchers built an apparatus to measure evaporation rates of a common water–polymer solution (polyvinyl alcohol, or PVA) at different humidities. They drilled five holes into the walls of a cylindrical plastic reservoir that would be filled with the solution and connected a glass capillary tube to each hole. The tubes were rectangular, with an inner cross section of 0.2 × 4 mm, and they extended horizontally away from the reservoir. The researchers filled the reservoir with a PVA solution. To ensure that evaporation would only occur at the protruding ends of the tubes, they deposited an oil layer on the top surface of the solution. The reservoir sat on a scale, and the whole assembly sat in a humidity-controlled box. For humidity held at values ranging from 25% to 90%, the researchers monitored the water mass lost from the reservoir in experiments that lasted about 17 hours each.
In each experiment, the evaporation rate initially remained constant, for about three hours. The rate then began to fall, as predicted by Salmon’s theory, because a polymer layer built up at the solution–air interface. However, the existing theory did not account for two observations. First, the early-stage constant evaporation rate—before a polymer layer formed—did not decrease with increasing humidity. Second, after the initial three hours, the evaporation rate fell, as expected, but it was independent of humidity only for humidity values up to 80%. At higher humidities, the evaporation rate decreased with increasing humidity, which indicated that other forces came into play.
Examining the open ends of the glass tubes under a microscope provided a clue. At the solution’s outermost surface, a layer of material appeared to have buckled and pealed off from the walls. The researchers propose that this layer was a gel skin that covered a thinner, elastic polymer layer and that the combination of the two further reduced the ability of water molecules to reach the surface. This extra layer was not anticipated by Salmon’s theory, but calculations accounting for its effects explained both of the discrepancies with the theory. Titmuss says that a gel-like skin has also recently been observed at the liquid–air interface of respiratory droplets, which contain biopolymers, so similar effects may be present in the droplets.
Salmon finds the new work “remarkable.” He says the results provide strong motivation for developing new models that account for effects such as the elasticity of the gel-like film at the liquid–air interface.
–Rachel Berkowitz
Rachel Berkowitz is a Corresponding Editor for Physics Magazine based in Vancouver, Canada.
References
- M. Huisman et al., “Evaporation of concentrated polymer solutions is insensitive to relative humidity,” Phys. Rev. Lett. 131, 248102 (2023).
- J.-B. Salmon et al., “Humidity-insensitive water evaporation from molecular complex fluids,” Phys. Rev. E 96, 032612 (2017).