Four Walls Good, Two Walls Bad for Confined Cells
One of the aberrant features of cancer cells is a failure to distribute chromosomes properly when the cells divide. Researchers have now found that a specific problem with the chromosome-distribution machinery can become more common in cancer cells confined within shallow microscopic channels—but also that, surprisingly, increasing the physical constraints can suppress these errors [1]. Such confinement mimics the effects of crowding by surrounding cells in a tumor, and the researchers believe the results might help to explain what goes awry in cancers and perhaps offer clues to how it might be put right.
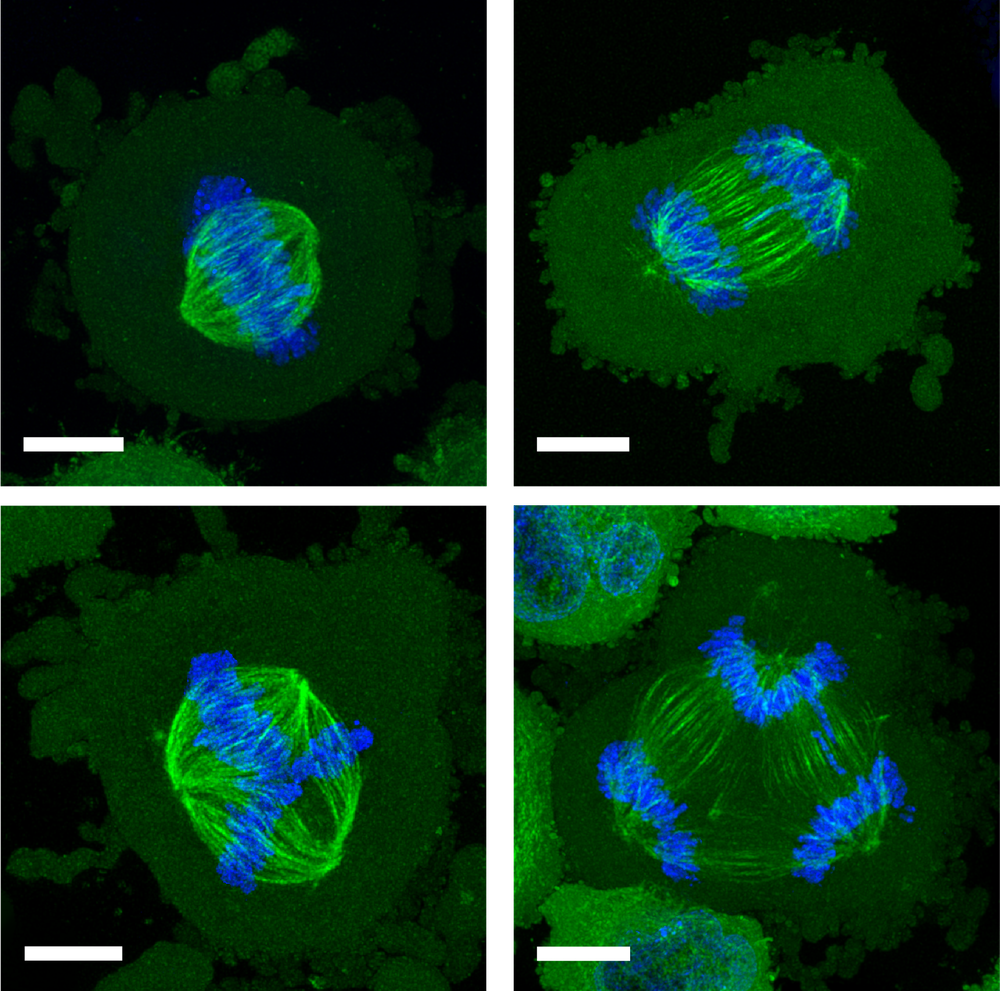
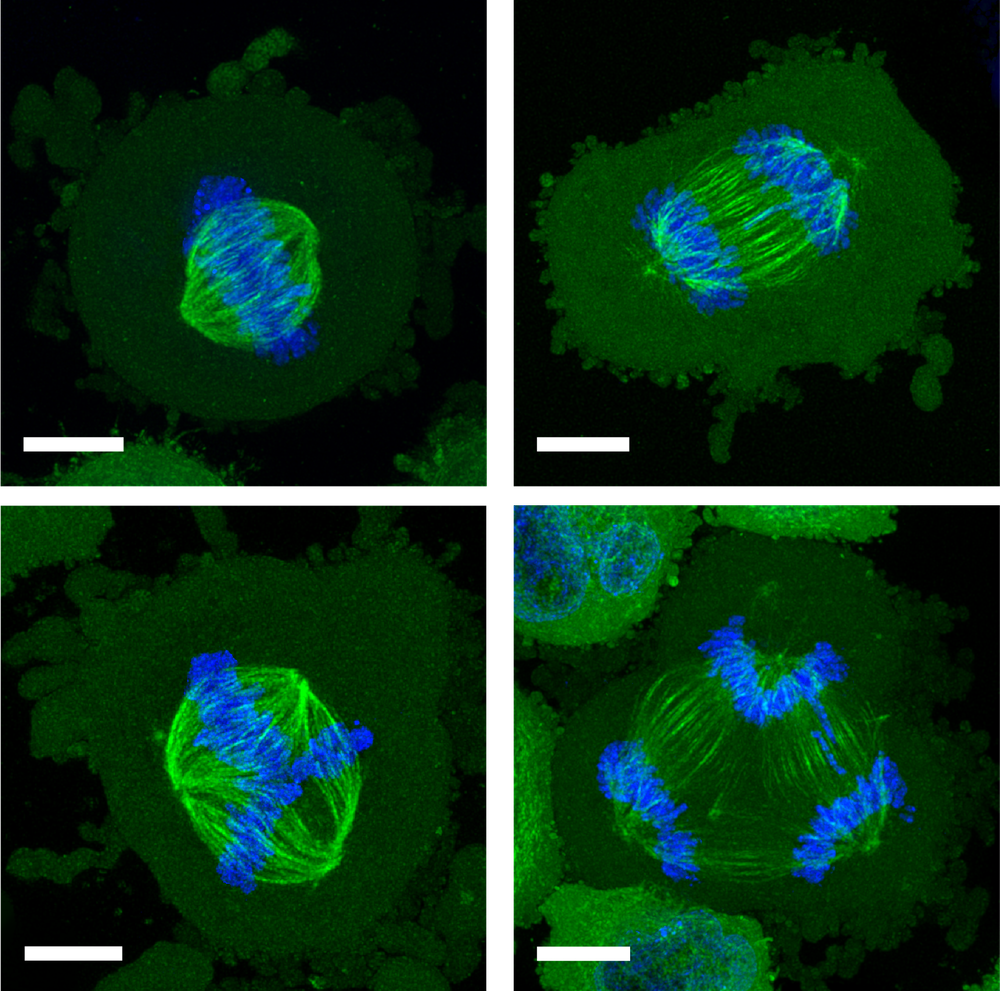
In a healthy, dividing cell, after the genome is replicated, the chromosomes are segregated into two groups. Both groups are bound to the mitotic spindle, a bundle of aligned filaments (called microtubules) that are pinched together at the ends into structures called poles. The chromosomes are then drawn along the microtubules toward the poles. A key cause of improper chromosome segregation in cancer cells is the formation of spindles with more than two poles. Multipolar spindle formation inside living organisms may differ from the phenomenon when observed in cells grown in a dish [2], so it is possible that the confining effect of the surrounding cells in a tissue has some influence on this process.
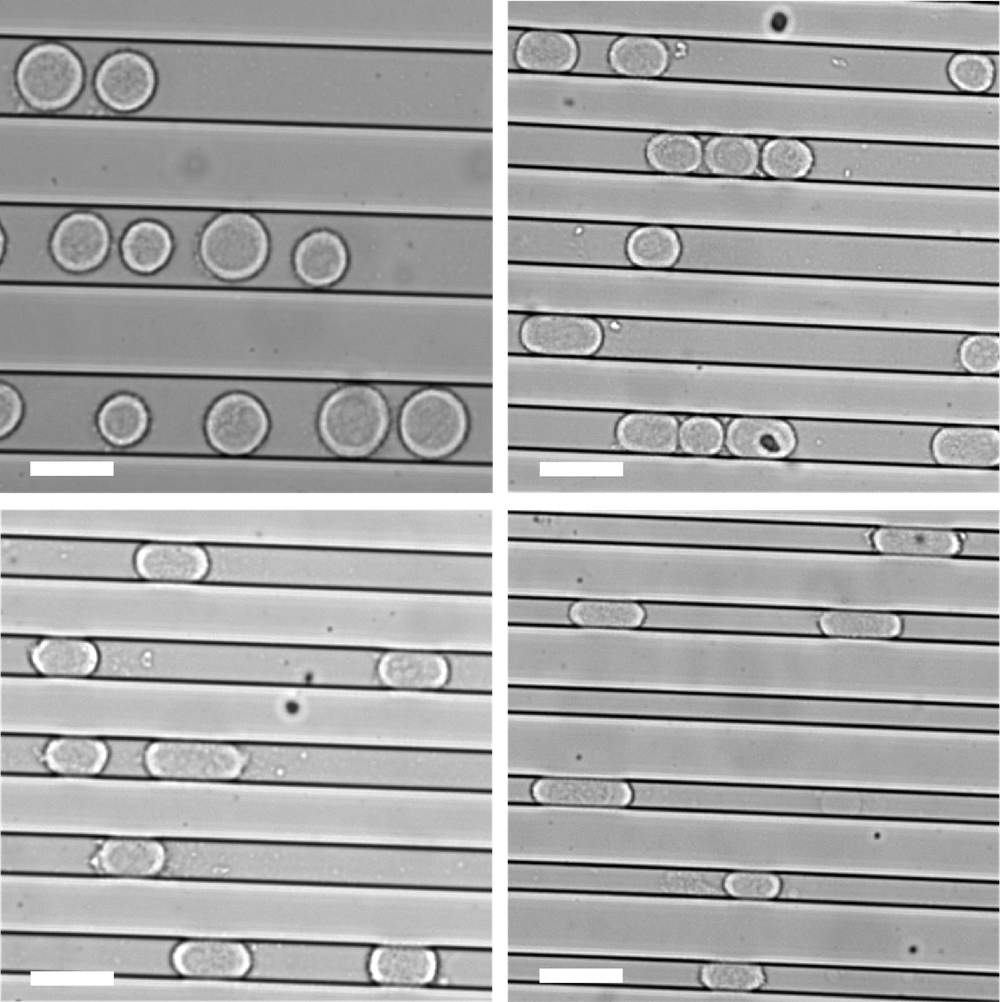
To investigate the effect of a confining environment, Hongyuan Jiang of the University of Science and Technology of China and colleagues grew individual human cancer cells within microscopic channels in a plastic slab placed channels-down on a glass slide. If the channels were wide and deep enough, the cells could grow without touching the walls. But if the channels were shallower than about 20 𝜇m, the cells were confined by the “floor” and “ceiling.” And if the channels were narrower than 20 𝜇m, the cells also pushed against the channel side walls. So channels less than 20 𝜇m in both width and depth confined the cells with four walls, forcing them to grow in an elongated shape.
These two modes of confinement—two-wall and four-wall—had strikingly different effects on the shapes of the cells’ mitotic spindles. When they grow free and unconfined, these cancer cells already show a significant incidence (about 12%) of spindles with three to five (or sometimes more) poles. But two-wall confinement increased this incidence to around 60% for channels 3 𝜇m deep. This result is consistent with earlier studies showing that such microconfinement of cancer cells can lead to the formation of multiple daughter cells, often with chromosomal abnormalities [3].
It is a different story for four-wall confinement in narrower channels. In 10- 𝜇m-deep channels, around 10% of the cells had multipolar spindles for channel widths of 22–30 𝜇m, but that proportion decreased to just 4% for widths of 8–12 𝜇m. That seems odd: if some confinement promotes multipolar spindle formation, why should more confinement suppress it?
Jiang and colleagues think it comes down to how readily a pair of poles merges or a single pole splits into two. They devised a simple model to explore these processes, in which poles are treated like particles that can repel or attract one another. The model also takes account of interactions between the poles and the inner surface of the cell membrane. These interactions are crucial in the case of four-wall confinement, which forces the cells to elongate and thus brings the poles, on average, closer to the cell membrane. This proximity to the membrane changes the energy balance of pole–pole interactions to make pole clustering more likely and pole splitting less likely, thereby reducing multipolarity.
“The problem they addressed is very important for biomedical applications, as many cancer cells, and even healthy cells, assemble multipolar spindles and divide incorrectly,” says mathematical biologist Alex Mogilner of New York University. “This is pretty much the first study that directly demonstrates how crucial the cell shape is. It is a great example of a bottom-up physical approach to a complex biological problem.” At the same time, the findings “highlight how little we know about spindle multipolarity,” says biophysicist Jonathan Howard of Yale University.
Whether the results will lead to new ways of suppressing tumors remains to be seen. Soft-matter physicist Joachim Rädler of Ludwig Maximilian University of Munich cautions that it is notoriously difficult to translate what is learned from lab-grown cells into human therapies. But Jiang is optimistic. “Multipolar spindles promote cancer progression to more malignant forms,” he says, so controlling the degree of confinement of cells in a tumor “would be a potential strategy for cancer prevention and therapy.”
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- L. Cheng et al., “Appropriate mechanical confinement inhibits multipolar cell division via pole-cortex interaction,” Phys. Rev. X 13, 011036 (2023).
- K. A. Knouse et al., “Chromosome segregation fidelity in epithelia requires tissue architecture,” Cell 175 (2018).
- H. T. K. Tse et al., “Increased asymmetric and multi-daughter cell division in mechanically confined microenvironments,” PLoS ONE 7 (2012).