X-Ray Spectroscopy of a Lone Atom
X-ray spectroscopy offers exquisite tools to study the composition and structure of samples relevant to biology, chemistry, environmental studies, and materials sciences. It can not only probe “fingerprints” of specific chemical elements in a sample but also reveal a wealth of information about the chemical states and the structural arrangement of atoms in the sample. Until now, however, experiments needed thousands of atoms—or an attogram of sample—to obtain a clean enough signal. Now a team led by Saw-Wai Hla of Ohio University and Argonne National Laboratory in Illinois has been able, for the first time, to measure the x-ray spectra of single atoms [1]. The breakthrough was achieved with a technique that combines x-ray spectroscopy with the premier single-atom-resolving technique—scanning tunneling microscopy (STM).
Developed in 1981, STM has gotten us used to remarkable views of the microscopic world. A decade ago, IBM researchers even realized the world’s “smallest” movie, A Boy and His Atom, whose frames were made up from molecules moved around with an STM tip. STM, however, doesn’t deliver information on the atomic species. “I routinely image and manipulate atoms with STM. But with STM alone I couldn’t identify what types of atoms are in an unknown sample,” says Hla.
The lack of elemental specificity of STM results from the fact the tunneling current measured with the STM tip involves the easiest electrons to strip away from an atom—its outermost electrons, whose features aren’t element specific. X-ray spectroscopy, on the other hand, targets an atom’s core electrons, which can offer unique fingerprints in the form of element-specific absorption lines. What’s more, details of these absorption lines can reveal information about the atom’s chemical state, such as how it may be sharing electrons with its neighbors. The x-ray absorption of a single atom, however, is so weak that many atoms are typically needed to produce a measurable signal on a detector.
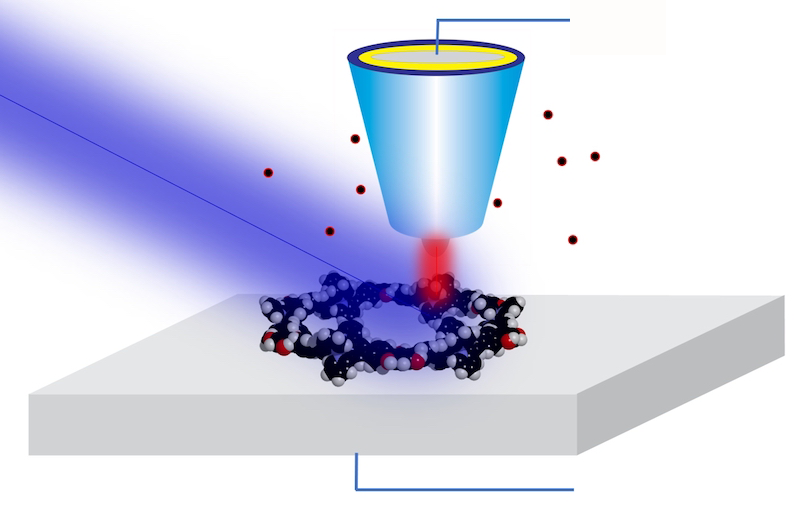
For years, Hla and his team have worked to combine the best of both worlds—STM’s atomic resolution and x-ray spectroscopy’s chemical sensitivity. Their approach makes use of a previously demonstrated technique called synchrotron x-ray STM (SX-STM). In the technique, a sample is scanned by an STM tip while being exposed to the x-ray beam delivered by a synchrotron. The tip records a large tunneling current when the x-ray energy is resonant with core-level transitions in the sample’s atoms. By measuring the current as the x-ray energy is varied, researchers can recover an x-ray absorption spectrum of the sample below the tip.
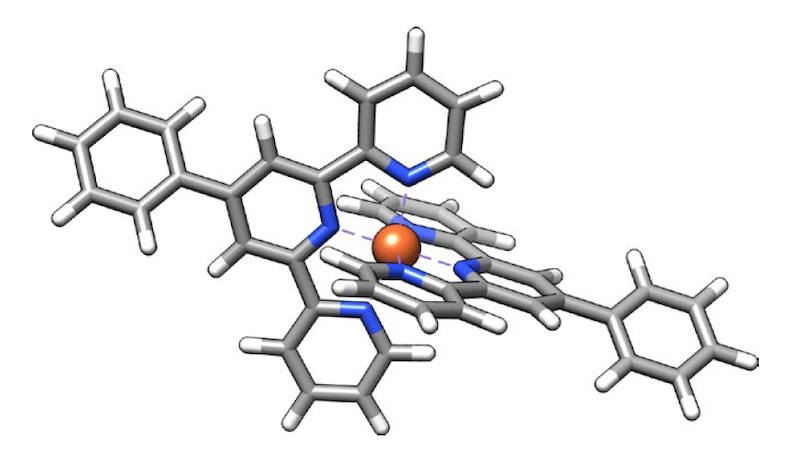
While SX-STM has been available and applied to nanoscale imaging since 2009 [2], bringing the resolution down to single atoms was anything but straightforward, says Hla. A key challenge was to prepare a suitable sample in which a specific atom could be singled out from its environment to be reliably measured. To do so, the team synthesized special supramolecular complexes—which allowed a single atom to be placed in a controlled and reproducible position.
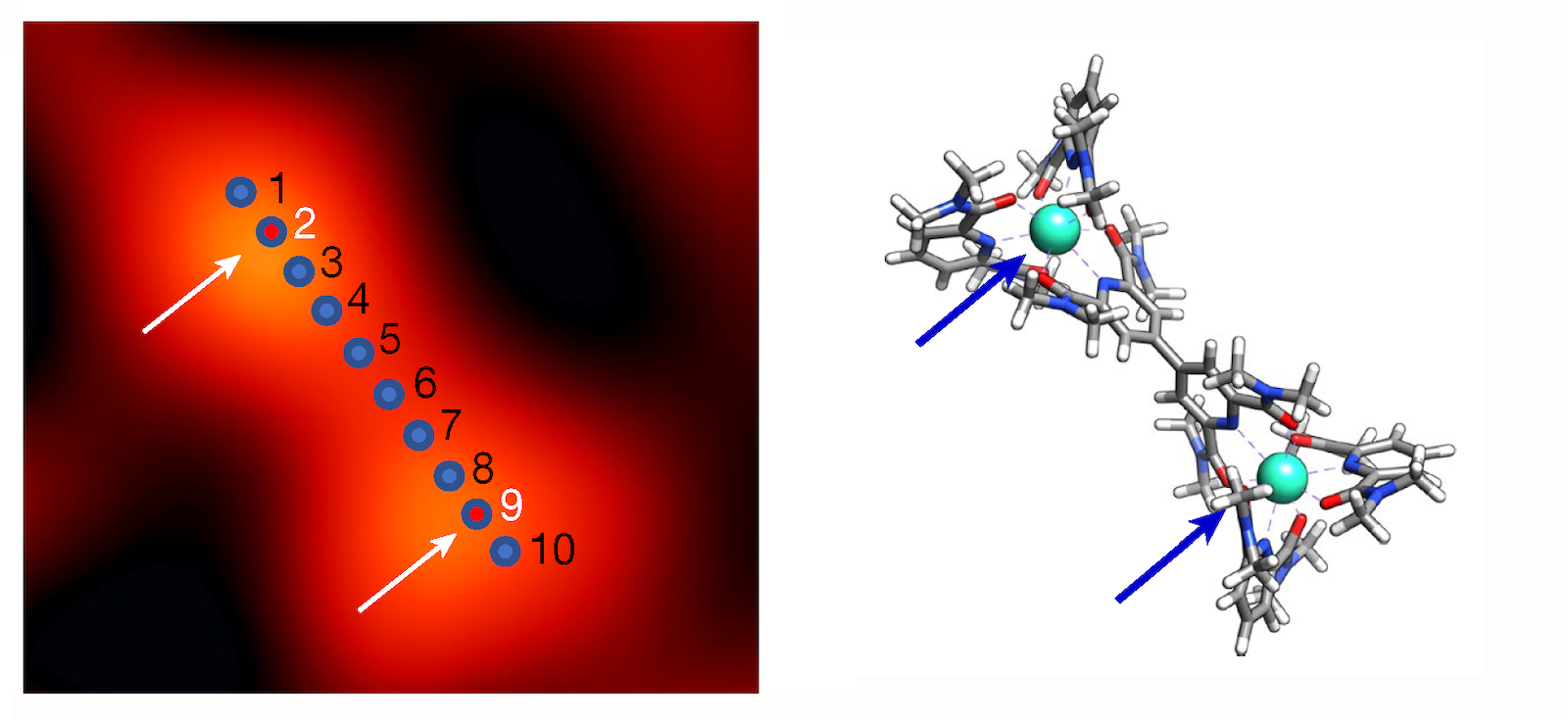
In the new work, the team studied two different elements, iron and terbium, which were each placed in a supramolecular complex that isolated the target atoms from other atoms of the same species. This design ensured that the measured tip current could be unambiguously ascribed to a single atom. As they scanned, for example, their tip along the length of a complex containing two terbium atoms, the researchers recorded x-ray spectra consistent with terbium’s “M edge” (which involves transitions of core electrons from 3d to 4f states). In a second experiment on another supramolecular complex, they detected iron’s “L edge” (which involves 2p-to-3d transitions) at points within an iron-carrying complex.
Beyond identifying the iron and terbium atoms in the samples, the results also showcased the potential for revealing subtle chemical details. The analysis of the spectra confirmed, for instance, that iron is in a +2 oxidation state and that it strongly interacts with its surroundings (technically speaking, the atom’s d orbitals mix, or hybridize, with the p orbitals of six neighboring nitrogen atoms). For terbium, the team confirmed an expected property—the isolation from its surroundings due to the lack of hybridization of its f orbitals. This weak coupling is key to the many technological applications of terbium and other rare earths.
“The idea of doing x-ray spectroscopy on a single atom is quite extraordinary, and to perform such a measurement correctly is an amazing tour de force,” says condensed-matter physicist Michael Crommie of the University of California, Berkeley, who wasn’t involved in the study. “The demonstrated sensitivity for atoms spaced only a few angstroms apart is simply amazing,” says Thomas Jung, an experimental physicist at Paul Scherrer Institute in Switzerland. Such single-atom resolving power could help in understanding and designing so-called metal–organic coordination networks—materials that hold promise for applications from surface catalysis to quantum technologies, he says.
Hla says his team is working on further improving the setup. In particular, the researchers aim to carry out measurements using polarized x rays. This approach would make the experiment sensitive to a single atom’s spin state, which would be invaluable for studying rare-earth-based materials that have tantalizing applications in spintronics and magnetic memory technology.
–Matteo Rini
Matteo Rini is the Editor of Physics Magazine.
References
- T. M. Ajayi et al., “Characterization of just one atom using synchrotron X-rays,” Nature 618, 69 (2023).
- T. Okuda et al., “Nanoscale chemical imaging by scanning tunneling microscopy assisted by synchrotron radiation,” Phys. Rev. Lett. 102, 105503 (2009).