Tension Remodeling Resolves Tissue Architecture Question
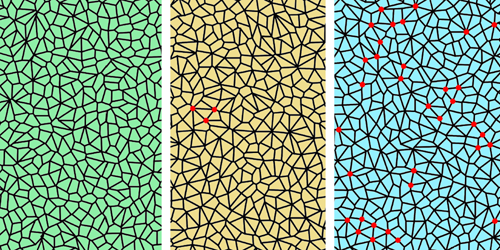
Epithelial tissues line the surfaces of every organ in our bodies. In the earliest stages of organ development and in wound healing, the cells that make up these simple sheets constantly rearrange themselves, exchanging positions like molecules in a liquid. But this fluidization is often hindered by the formation of multicell clusters, whose origins remain unclear. Using a dynamical structural model, Fernanda Pérez-Verdugo and Shiladitya Banerjee of Carnegie Mellon University in Pennsylvania now identify the mechanical prerequisites that lead to the formation and dissolution of these stabilized clusters [1]. They show how dynamic feedback between tension and strain controls the tissue’s material properties.
Existing models of tissue fluidity treat epithelial tissues as foam-like, polygonal networks of cells whose edges join at triple points. However, these models fail to explain the mechanisms underpinning cell neighbor exchanges. In particular, they oversimplify such exchanges by treating them as an instantaneous process, thereby avoiding the impact of exchanges that stall midprocess. One resulting discrepancy with experimental results is the absence of stable “rosette” structures that are observed in developing tissues where four or more cells meet.
To reproduce stalled exchanges in their model, Pérez-Verdugo and Banerjee made the tension across cell boundaries strain dependent. Specifically, cell junctions remodel themselves so that when the tissue undergoes local extension, tension is minimized, and when it undergoes contraction, tension is increased. They found that this strain–tension feedback allows the spontaneous assembly of stable, flow-suppressing cellular rosettes that dissolve when the tension remodeling is inhibited. The researchers say that the patterns that emerge in their model mirror cellular structures observed in living tissues, suggesting that this remodeling process is responsible for regulating tissue morphology.
–Rachel Berkowitz
Rachel Berkowitz is a Corresponding Editor for Physics Magazine based in Vancouver, Canada.
References
- F. Pérez-Verdugo and S. Banerjee, “Tension remodeling regulates topological transitions in epithelial tissues,” PRX Life 1, 023006 (2023).