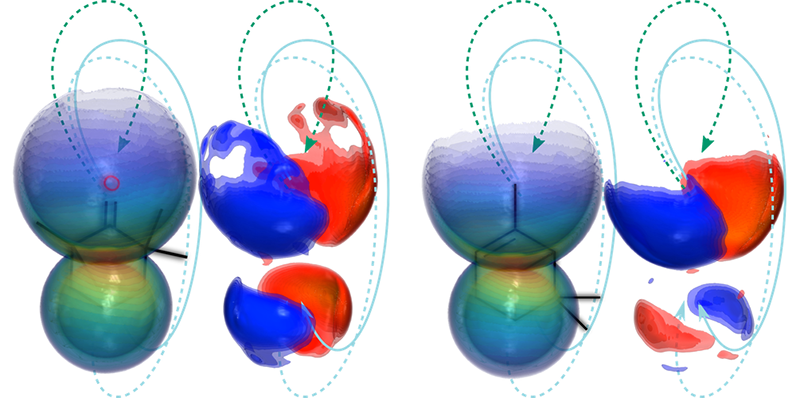
Probing Chiral Molecules with Their Own Electrons
Cook up a batch of amino acids—molecular building blocks of life—and only half of the molecules will come out as planned. That’s because each amino acid comes in two “chiral” forms, one that twists like a right-handed screw and one that twists like a left-handed screw. Any attempt to make one form always produces an equal amount of the other unless some chiral-selective substance is included in the mix, as happens in nature: living creatures only contain left-handed amino acids; the right-handed versions are not conducive to life.
Exactly why that asymmetry exists remains unanswered, as do other questions related to the behavior of chiral molecules. The problem is that chirality remains a tricky property to probe experimentally. Now Debobrata Rajak and Yann Mairesse from the National Centre for Scientific Research (CNRS) in France, and their colleagues have developed a technique for determining the chirality of a molecule using the molecule’s own electrons [1]. The technique could allow researchers to monitor the dynamics of chiral molecules on attosecond timescales, providing a new view of these twisty objects.
Methods exist for probing the chiral properties of a set of molecules in a concentrated form, such as in a powder or a liquid. But in those states, interactions among the molecules can influence measurement outcomes. Monitoring gas-phase molecules solves this problem, and two decades ago theorists proposed a way to distinguish the chirality of such molecules using electrons. That proposal called for aligning the orientations of the molecules relative to the electron-beam direction, a tricky condition to meet if the molecules can float. “There’s been a lot of progress in developing methods to align gas molecules, but it remains something that is an experiment by itself,” Mairesse says.
Rajak, Mairesse, and their colleagues solved this issue by imaging the molecules with their own electrons, ensuring alignment between each molecule’s orientation and its probe electron’s trajectory. The team studied left- and right-handed versions (enantiomers) of two organic chiral compounds: fenchone, which is found in absinthe and fennel, and -pinene, which is present in eucalyptus leaves and orange peels. In separate trials, they placed one enantiomer in vapor form inside a vacuum chamber and then shone an intense elliptically polarized femtosecond pulsed laser at the system.
The strength of the pulses and their orientation relative to the molecules were such that each pulse kicked out one electron from a subset of the molecules. The elliptical shape of the pulses put the freed electrons on a course that accelerated them away from their parent molecules and then drove them back. But the returning electrons didn’t return to their starting point. Rather, they were diffracted by electrostatic interactions with the ionized molecules and directed toward a detector that recorded their properties.
The team repeated the ionization-detection steps 72 times for 72 different orientations of the laser-pulse polarization relative to the detector, allowing them to reconstruct the full three-dimensional electron-diffraction pattern of molecules, from which they retrieved the molecules’ structure. They then removed all the molecules from the chamber and refilled it with a gas of the opposite enantiomer of that same compound and repeated the experiment.
Comparing signals from the different experiments, Rajak, Mairesse, and their colleagues found that the right- and left-handed versions of each compound preferentially diffracted the electrons in opposite directions relative to the direction of the laser pulses. This asymmetry reached several percent and provided the “smoking-gun” evidence that the experiments were monitoring chiral properties of the molecules rather than an artifact, Mairesse says. The researchers were able to uncover this signal in part thanks to shielding that kept out stray magnetic fields that can divert the paths of the electrons after ionization, destroying the chiral signals. “Even Earth’s magnetic field can influence the outcome,” Rajak says. “The chiral signals we are measuring are very subtle.”
The team says that another key to this successful demonstration was the femtosecond laser. It provided the strong electric field needed to tear the electrons from the atoms and produce the diffraction effect. The high repetition rate of the pulses also provides the possibility of observing the time-dependent chiral behavior of a molecule, for example, during a photochemical process. The positions of a molecule’s nuclei relative to one another determine its chirality, and these positions can change on a timescale of a few tens to a few hundreds of femtoseconds during a chemical reaction. Our technique could certainly be used for such measurements, Mairesse says.
Molecular chirality plays a decisive role in determining the outcomes of chemical reactions, says Andrés Ordóñez, a theoretical physicist at Imperial College London who specializes in developing techniques for imaging and manipulating chiral molecules. For him, the team’s use of recolliding electrons to yield key three-dimensional structural information about a molecule is an “impressive” advance that reveals a new chiral phenomenon. “This work will surely motivate further fundamental research on using similar techniques to image the ultrafast dynamics of complex molecules.”
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- D. Rajak et al., “Laser-induced electron diffraction in chiral molecules,” Phys. Rev. X 14, 011015 (2024).