Lithium-Ion “Traffic Jam” Behind Reduced Battery Performance
This article is part of a series of pieces on advances in sustainable battery technologies that Physics Magazine is publishing to celebrate Earth Week 2024. See also: Q&A: Electrochemists Wanted for Vocational Degrees; Q&A: The Path to Making Batteries Green; News Feature: Sodium as a Green Substitute for Lithium in Batteries; Research News: A New Cathode for Rechargeable Magnesium Batteries.
Electric vehicles are picking up visibility in the public eye. But their adoption is slowed down by batteries that degrade over time, an issue commercial ventures are especially keen on addressing as they adopt increasingly nickel-rich cathodes—the cathode du jour for high-end electric vehicles. The substitution of nickel for cobalt in earlier versions of these cathodes can improve their performance, but it also accelerates degradation. Earlier this year, Louis Piper, University of Warwick, UK, and his colleagues devised and demonstrated an x-ray technique that can examine industry-grade versions of nickel-rich lithium-ion batteries in real time [1]. Their observations help to narrow down why these batteries degrade and lead to suggestions for how to prolong battery lifespans.
The first commercially successful cathode for a lithium-ion battery consisted of layers of lithium alternated with cobalt oxide. This cathode delivered a current of 140 milliampere hours per gram (mAh/g) of material, which indicated it operated using only 50% of the lithium in the cathode. That finding led to a decades-long race to substitute the cobalt for nickel, as predictions indicated that would increase availability of lithium in the cathode to 70% and therefore increase the battery’s practical energy capacity. The switch would also avoid the need for cobalt, which is toxic and is associated with an unethical supply chain. But reaching the predicted energy capacity of 200 mAh/g with lithium-nickel batteries has proved tricky because of increased degradation of the cathode. “The big problem is closing the gap between theoretical and practical [energy] capacity of the layered oxide cathodes,” Piper says.
Progress is being made. For example, Tesla’s new 4680 cylindrical batteries use a cathode containing 80% nickel. These batteries have a high electrochemical performance, a direct result of the nickel-rich layer allowing more lithium ions to flow. Additionally, the design appears to benefit from the ability of single-crystal nickel-rich cathodes to mitigate stresses that have previously caused other cathodes to crack and degrade.
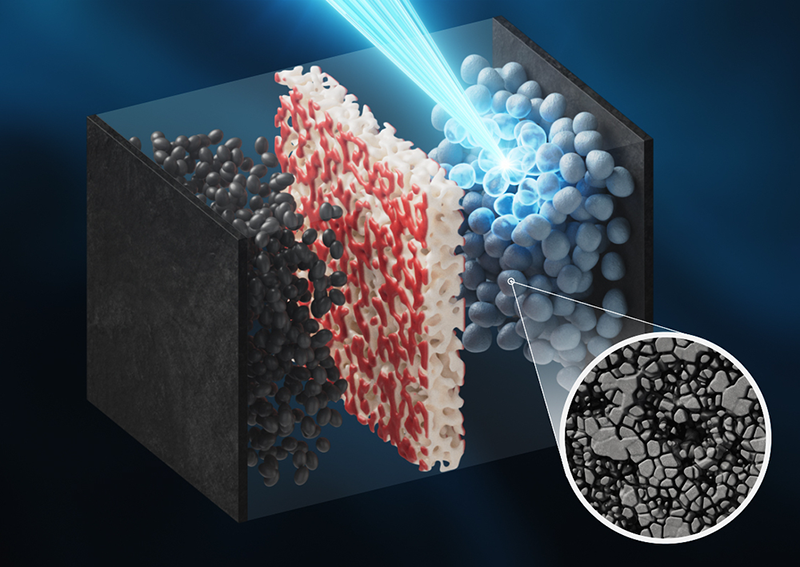
But these batteries still degrade. Post-use microscopy studies of such batteries hint that degradation comes as oxygen leaches out of the nickel-rich cathodes. To tease out exactly why these batteries degrade, researchers would like to watch what happens inside a battery as it undergoes charging and discharging cycles. This feat has proved tricky to complete because researchers generally cannot access the reactions within full-scale versions of the batteries, as the batteries are multilayer sandwiches with many buried interfaces, which makes it hard to probe the system with standard laboratory setups.
In their experiments, Piper and his team studied industry-grade nickel-rich lithium-ion batteries that they manufactured on a precommercial production line. The batteries were subjected to accelerated charging–discharging cycles. The researchers integrated the batteries into x-ray diffraction machines and exposed them to the aggressive voltages, charging speeds, and charging depths (the “fullness” of the battery charge) experienced by commercial batteries.
The real-time x-ray data collected during the charge–discharge cycles show that the surface structure of the nickel-rich cathodes underwent chemical changes at high voltages. The team found that the nickel-rich surfaces lost oxygen, while the bulk oxidized. This change caused the buildup of an oxygen-poor surface layer that trapped lithium ions, resulting in a capacity drop of 10% after 100 cycles. “You end up with a reduced rock-salt nickel oxide crust, about 2 to 5 nm thin, that acts as a kinetic trap slowing lithium extraction and insertion,” Piper says. Further experiments indicated that slowing the rate of charging and discharging could alleviate the lithium “traffic jam,” though not entirely reverse it.
“We can draw many conclusions about how a battery is degrading from electrochemical data,” says Yulia Preger, a chemical engineer at Sandia National Laboratories, New Mexico. But “materials characterization approaches like this can give new insights to help us design better materials to make better batteries.”Jacqueline Edge, a battery scientist and physicist at Imperial College London, agrees. Piper proposes that doping or coating the cathode surface with aluminum or aluminum oxide could help to passivate the nickel surface and prevent lithium traffic jams. “The new in-lab [measurement] methodology provides a means of validating designs faster for physics-led industrialization,” she says.
–Rachel Berkowitz
Rachel Berkowitz is a Corresponding Editor for Physics Magazine based in Vancouver, Canada.
References
- A. S. Menon et al., “Quantifying electrochemical degradation in single-crystalline LiNi0.8Mn0.1Co0.1O2–graphite pouch cells through operando x-ray and postmortem investigations,” PRX Energy 3, 013004 (2024).