Atomic wires of carbon
Carbon has an amazing number of different atomic configurations. Apart from the crystalline forms of diamond and graphite, we are now familiar with the cage structures of the fullerenes [1], the elongated carbon nanotubes [2], and the two-dimensional sheets of graphene [3], and each of these forms has led to a wealth of scientific discoveries and a range of applications. In a paper in Physical Review Letters, Chuanhong Jin, Kazu Suenaga and Sumio Iijima at NIAIST in Tsukuba, Japan, and Haiping Lan and Lianmao Peng at Peking University in China show that with a high-energy electron beam, they can transform graphite into graphene, and further into separate strings of carbon atoms [4]. There have been only scarce reports on carbon atom chains, and their new approach adds an avenue to produce new all-carbon electronic nanostructures.
Carbon is surprisingly versatile in its bonding configurations, which explains its important role in producing the multitude of compounds that make up all living organisms. Even in its pure form, carbon is capable of binding into structures as diverse as diamond, graphite, fullerenes, nanotubes, graphene, and now, ultimately, as strings of atoms. All of the noncrystalline forms of carbon have been discovered by nonstandard approaches. Fullerenes were first observed in cluster-beam experiments, nanotubes were found in carbon soot by transmission electron microscopy, and graphene was produced by exfoliating graphite with Scotch tape. While short strands of carbon chains have been produced by chemical synthesis [5], the method described by Jin et al. is more in line with the unconventional approaches for making other carbon nanostructures and may provide an avenue for integrating the chains into graphene (or graphite) based electronic nanodevices. Their method is also similar to one used to produce and observe metallic chains of gold atoms [6].
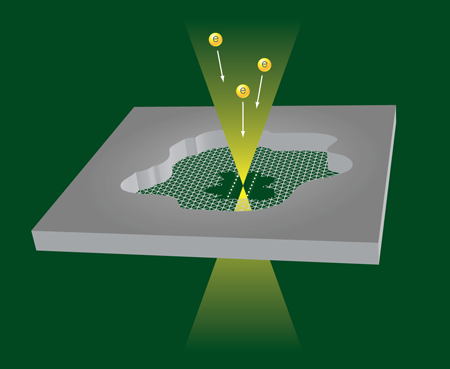
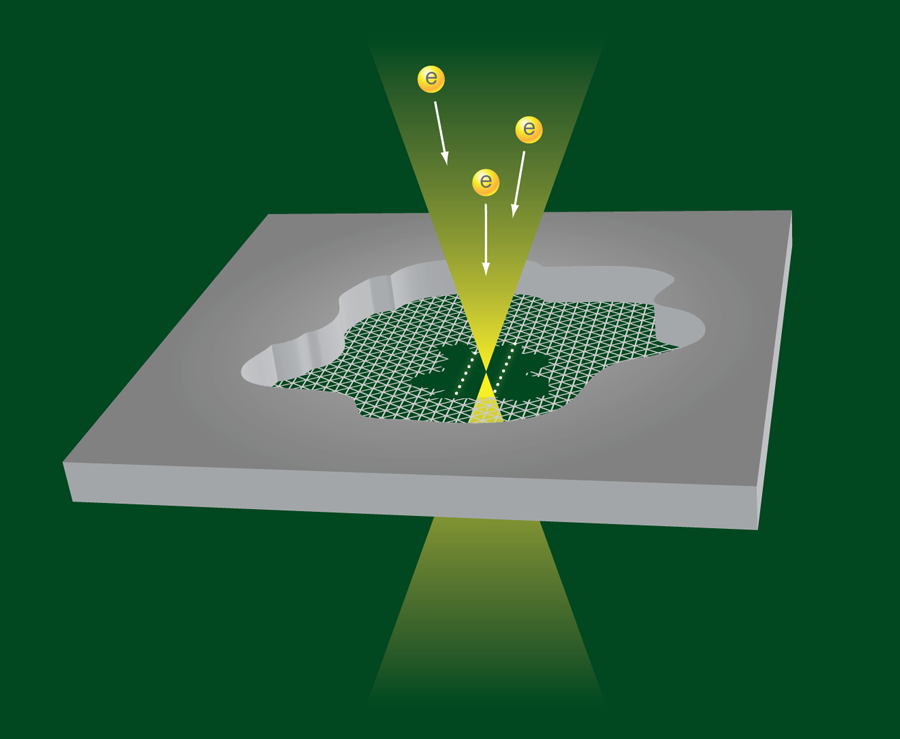
To make the structures, Jin et al. manipulate the electron beam in a transmission electron microscope. They start with a small flake of graphite imaged by means of the electron beam optics. By focusing a high-energy, high-current beam on a spot on the flake, they remove carbon atoms and thin the flake until they expose a single atomic carbon layer (i.e., graphene) (Fig. 1). Further irradiation at high energy and intensity produces two neighboring holes in the graphene layer, separated by a graphene nanoribbon, which they can continue to thin with more irradiation. Jin et al. argue that the energy of the edge states of the ribbon is so much higher than those at the center of the ribbon that the edge atoms will be preferentially removed in the process. At the last stage of narrowing the nanoribbon, when the two edges come together, the ribbon can be seen to break up into two parallel single-atom strands, as is beautifully illustrated in Video 1 . In other runs of the experiment, single carbon strands are formed. The atomic chains are surprisingly stable under the harsh conditions of electron beam irradiation, and have been observed to survive for more than 100s.
Free standing carbon atomic chains have been observed previously [7,8], but the present experiments demonstrate a transformation from graphite to graphene, from graphene to a graphene nanoribbon, and finally from the nanoribbon to atomic chains. The breakup into two parallel chains is explained in terms of the total energy for the chains, which is lower than that for a narrow nanoribbon with two edges having high-energy edge states. Jin et al. support this idea with density-functional-theory calculations. The observed chains are also longer than what has been previously observed, up to 2.1nm, or 16 carbon atoms in a row. The interatomic distance of the carbon atoms in the chain cannot be resolved in the images, which leaves unanswered the question of whether the group is observing cumulene (all carbons connected by double bonds) or rather polyyne (alternating single and double bonds). While the authors do not draw any conclusions regarding this issue, some bond alteration appears to be visible in the results of the calculations in the auxiliary material of their paper, and this seems to depend on whether the number of carbon atoms in the chain is even or odd.
Are carbon atom chains conducting? The approach by Jin et al. does not permit this question to be addressed experimentally. From density-functional theory, several groups [9,10] have made predictions that carbon atomic chains should be nearly perfect molecular wires, irrespective of the presence of bond alteration. Yuzvinsky et al. [7] reported a first attempt at conductance measurements on carbon chains, also using a transmission electron microscope, but the measurement was obtained on a transient, unstable chain structure that could not be clearly resolved. The conductance was observed to be an order of magnitude lower than predicted, perhaps suggesting that the structure obtained was not a carbon chain.
Scanning tunneling microscopes, or break junction techniques, have been exploited with great success in analyzing metallic atomic chains by two-probe electrical conduction experiments [11]. As the method reported by Jin et al. for producing carbon atomic chains is similar to that used to produce atomic chains for gold, why have we not seen any reports of similar experiments using STM or break junctions on the formation and measurement of carbon chains? The answer is probably in the difference in bonding configurations. For metals the formation of chains in these experiments relies on the plastic deformation of a metallic nanowire in a process of stretching. Atoms need to have a high degree of freedom to switch between bonding configurations. The two-dimensional nature of the graphite lattice limits this freedom dramatically, and further limitations are imposed by the higher degree of directional bonding for carbon as compared to metals. Moreover, the individual graphene layers in a graphite flake are likely to produce many parallel conduction paths, where each layer would break at a different spot and at a different instance, resulting in irrecoverable masking of the individual atomic junction properties. Producing an atomic chain between two electrodes from single-layer graphene or from a single carbon nanotube is a challenge that, perhaps, can be met, but will require great ingenuity.
References
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, and R. E. Smalley, Nature 318, 162 (1985)
- S. Iijima, Nature 354, 56 (1991)
- K. S. Novoselov et al., Science 306, 666 (2004)
- C. Jin, H. Lan, L. Peng, K. Suenaga, and S. Iijima, Phys. Rev. Lett. 102, 205501 (2009)
- A. D. Slepkov et al., J. Chem. Phys. 120, 6807 (2004)
- H. Ohnishi, Y. Kondo, and K. Takayanagi, Nature, 395,780 (1998)
- T. D. Yuzvinsky et al., Nano Lett. 6, 2718 (2006)
- H. E. Troiani et al., Nano Lett. 3, 751 (2003)
- N. D. Lang and Ph. Avouris, Phys. Rev. Lett. 81, 3515 (1998); ibid. 84,358 (2000)
- Z. Crljen and G. Baranoivc, Phys. Rev. Lett. 98, 116801 (2007)
- N. Agraït, A. Levy Yeyati, and J. M. van Ruitenbeek, Phys. Rep. 377, 81 (2003)