The Simplest Molecular Anion
For decades, the negative molecular ion, , was so elusive that researchers wondered whether it could hold together at all. About five years ago, researchers finally got clear evidence this molecule can form, and now, in Physical Review Letters, they describe its structure and exactly how it falls apart.
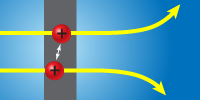
To get a snapshot of the short-lived ions, Brandon Jordon-Thaden of the Max Planck Institute for Nuclear Physics in Heidelberg, Germany, and his colleagues use a technique called Coulomb explosion imaging. They fire a beam of the ions through an ultrathin carbon foil, which strips away all three of the ions’ electrons in a fraction of a femtosecond, faster than the nuclei can respond. Once an ion’s two nuclei are stripped bare, their positive charges rapidly push them apart. By measuring how far different pairs fly apart as they travel several meters to a detector, the team deduces the range of separations the nuclei had in the original ions: the closer the nuclei start, the further away they push each other.
In the most stable ions, the nuclei orbit each other rapidly at a separation of about six atomic units, several times that in a neutral molecule. But some paired nuclei appear to have started out much closer. The researchers conclude that these close pairs hit the foil as neutral hydrogen molecules that had formed in the beam, when unstable molecular ions rejected their extra electron. – Don Monroe