Heavy into Stability
In 1940, the first synthetic element heavier than uranium—neptunium-239—was produced by bombarding uranium with neutrons. Since then, nuclear scientists have ventured into the search for new heavy elements, expanding the frontiers of the physical world. The creation of elements with atomic number beyond that of uranium is challenging, as the half-life of elements decreases with increasing atomic number. However, nuclear theories have predicted that a so-called “island of stability” exists for certain superheavy elements of the nuclide chart, which should have half-lives ranging from minutes to many years.
The search for this island of stability has led to the creation of elements with up to 118 protons. The last element to be discovered was 117 [1] (see 9 April, 2010 Viewpoint), filling in the final gap on the list of observed elements up to element 118. Now, writing in Physical Review Letters, Yuri Oganessian at the Joint Institute for Nuclear Research (JINR), Russia, and colleagues report on a second production campaign for element 117 [2], which verifies their initial findings and provides a new comprehensive characterization of the decay chains of two isotopes of the 117 element. Their results confirm that we are indeed approaching the shores of the island of stability.
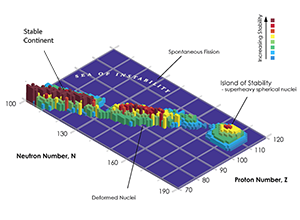
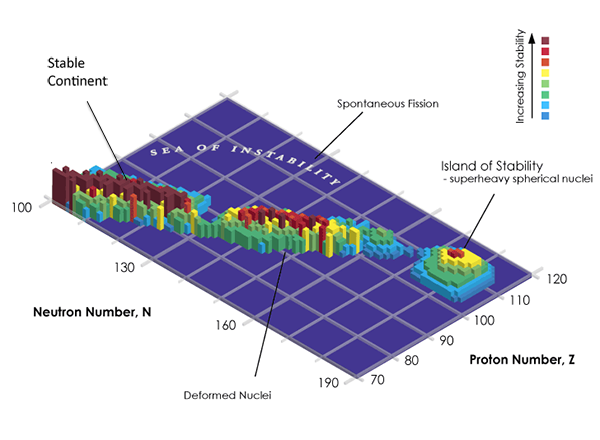
The stability of nuclides is a function of proton ( ) and neutron ( ) number, as illustrated in Fig. 1. A connected region (“continent”) of stable elements is found for lighter elements, ending at the lead–bismuth “cape.” All elements with an atomic number exceeding 82 (lead) are unstable, with decreasing half-life for higher atomic numbers. However, a first region of relative stability appears around the isotopes of thorium and uranium ( equal to 90 and 92, respectively) whose lifetimes are comparable with the age of the universe. Elements with atomic number greater than that of uranium (transuranium elements) have only been produced in laboratory experiments (see the historical review in Ref. [3]). The progress in this field is impressive: 26 new, manmade heavy elements have been synthesized within 60 years. Some of these elements (up to californium) can be produced in macroscopic quantities in nuclear reactors, using neutron capture processes to form heavier elements from actinides.
Elements beyond uranium should become more and more unstable as they get heavier, as Coulomb repulsion starts to be stronger than the strong force that holds the nucleus together. But in the late sixties, Glenn T. Seaborg postulated the existence of a relatively stable region of superheavy elements, an island of stability. This idea is based on the nuclear shell model, which describes the atomic nucleus as made of shells, similar to the well-known electronic shell model for atoms. Nuclear theorists, including myself [4,5], predicted that the stability of nuclei with so-called closed proton and neutron shells should counteract the repelling Coulomb forces. In isotopes with so-called “magic” proton and neutron numbers, neutrons and protons completely fill the energy levels of a given shell in the nucleus. Those particular isotopes will have a longer lifetime than nearby ones. According to theory, this second island of stability should be located around proton number or and neutron number . Reaching this island of stability would open new horizons in nuclear physics and technology, enabling the production of superheavy nuclides in macroscopic quantities and with sufficiently long half-life to carry out actual experiments. This would allow us to test our understanding of nuclear matter and to possibly exploit such long-lived elements for applications in medicine or chemistry.
Substantial progress in the synthesis of superheavy nuclei was achieved at the GSI Helmholtz Centre for Heavy Ion Research in Germany, where the elements with to have been synthesized for the first time in fusion reactions of heavy projectiles (from iron to zinc) with lead and bismuth targets [6]. Unfortunately, in these projectile–target combinations only the proton-rich isotopes of superheavy elements with very short half-lives can be produced, as they lie outside the island of nuclear stability. Within the last ten years, researchers at JINR have successfully synthesized six new heavy elements with – by following a different approach: instead of a heavy projectile, a high-intensity beam of lighter atoms (calcium-48) is aimed at heavy actinide targets made of uranium or transuranium elements. The use of neutron richer calcium-48 allows the synthesis of nuclides with neutron number closer to that needed for stability.
Up until 2010, there was a gap between elements 116 and 118. The obstacle towards the production of element 117 was that the appropriate target material, berkelium-249 ( ) with 97 protons, has a short half-life of only 330 days. In 2009, several milligrams of were produced at Oak Ridge National Laboratory in the US—enough to prepare a target and to perform the first experiment for the synthesis of element 117 at JINR [1]. In early March of 2012, a new portion of , , was shipped again from Oak Ridge to JINR, where physicists started the second production campaign for the synthesis of element 117.
The results of this campaign, reported in the paper by Oganessian et al. [2], confirm that a reliable method for the production of 117 exists. The authors can now state with confidence that two isotopes of this element, and , have been synthesized and provide a comprehensive characterization of their decay properties. Two decay chains of and five decay chains of were detected. Oganessian et al. also observe a concomitant decay chain of element 118. This occurs because, at the time of the experiment, part of the target material had already decayed into californium-249, which can generate element 118 in a fusion reaction with calcium-48. The measured lifetimes of the 117 isotopes and other elements along its decay chain are long, lying in the millisecond-to-second range. This is consistent with shell-model predictions, confirming that these elements are indeed located at the southwest shores of the island of stability. The consistent results emerging from the two productions campaigns at JINR may get the authors close to laying claim on naming the new element.
What are the prospects of reaching deeper into the center of the island of stability? Although fairly long lived, the isotopes of superheavy elements produced in the experiments with calcium-48 are still neutron deficient: each isotope needs six to eight more neutrons to lie within the island. This occurs because heavier stable atoms must have a larger neutron/proton ratio that lighter atoms. Thus creating a heavy atom by fusion of two lighter ones inevitably leads to an atom that has too few neutrons and too many protons to be stable. One would then deduce that there is no way to the island of stability. However, pathways towards the center of the island of stability may exist. Recent theoretical studies carried out in my research group suggest that superheavy nuclei located at the top left side of the island of stability, formed in ordinary fusion reactions, could get rid of excess protons via decay [7]. Other alternatives to get to the right neutron number might exploit neutron capture, rather than fusion: such techniques would require the exposure of heavy elements, such as uranium, to very high neutron fluxes. Theory shows that this could be achieved in hypothetical small-scale underground nuclear explosions [8] or by using pulsed nuclear reactors of the next generation, if their neutron fluence per pulse is increased by about three orders of magnitude.
While the island of stability is now more firmly in sight, the jury is still out on what navigation plan will turn out to be successful.
References
- Yu.Ts. Oganessian et al., “Synthesis of a New Element with Atomic Number Z=117,” Phys. Rev. Lett. 104, 142502 (2010)
- Y. T. Oganessian et al., “Production and Decay of the Heaviest Nuclei 293,294 117 and 294 118,” Phys. Rev. Lett. 109, 162501 (2012)
- G. T. Seaborg and W. D. Loveland, The Elements Beyond Uranium (John Wiley and Sons, New York, 1990)[Amazon][WorldCat]
- S. G. Nilsson, S. G. Thompson, and C. F. Tsang, “Stability of Superheavy Nuclei and Their Possible Occurrence in Nature,” Phys. Lett. 28B, 458 (1969)
- U. Mosel and W. Greiner, “On the Stability of Superheavy Nuclei Against Fission,” Z. Phys. A 222, 261 (1969); Also in the Proposal for the Establishment of GSI: Frankfurt-Darmstadt-Marburg (1967)
- S. Hofmann and G. Munzenberg, “The Discovery of the Heaviest Elements,” Rev. Mod. Phys. 72, 733 (2000)
- V. I. Zagrebaev, A. V. Karpov, and W. Greiner, “Possibilities for Synthesis of New Isotopes of Superheavy Elements in Fusion Reactions,” Phys. Rev. C 85, 014608 (2012)
- V. I. Zagrebaev, A. V. Karpov, I. N. Mishustin, and W. Greiner, “Production of Heavy and Superheavy Neutron-Rich Nuclei in Neutron Capture Processes,” Phys. Rev. C 84, 044617 (2011)