Pushing a Fullerene through a Nanotube
Fullerenes are large molecular cages built entirely of carbon atoms, and researchers have been able to modify their properties by trapping other atoms inside the cage. Writing in Physical Review Letters, two theorists offer an analysis of a more recent invention, a fullerene containing a single molecule of water. They show that it responds in a surprising way to an electric field, allowing the whole structure to be driven in either direction through a narrow channel. Although it’s not completely clear why an object with no net charge should respond in this way, the researchers suggest that their discovery could have practical applications, such as delivering drugs by guiding molecules that carry them.
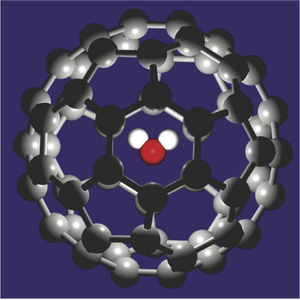
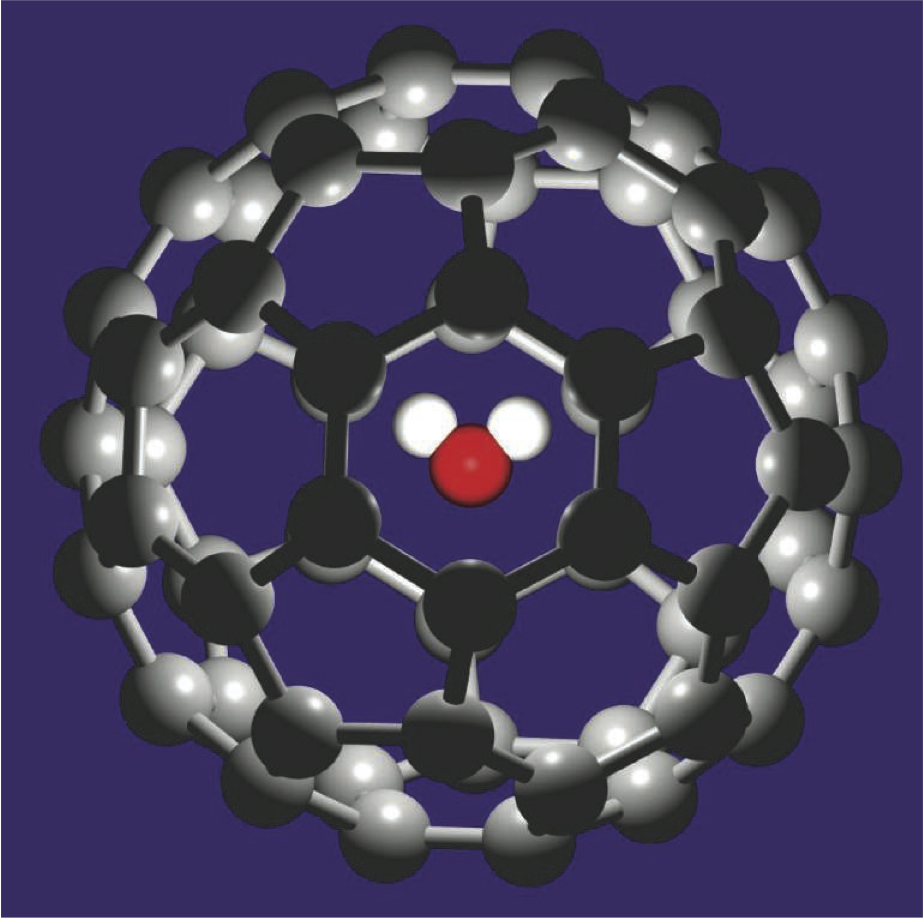
The most-studied fullerene is , a roughly spherical molecular shell made of carbon atoms. Two years ago researchers used organic chemistry “surgery” to open , insert a water molecule, and seal the incision, creating a structure designated @ [1].
Baoxing Xu and Xi Chen of Columbia University in New York City have now used computer simulations to explore the properties of this structure. Their simulation captures all of the interactions between the carbon atoms and the three atoms of the water molecule. The researchers treated the as a rigid structure, undistorted by its cargo, because the 1-nanometer-diameter cage is so much larger than . They then placed the simulated @ inside an 8.2-nanometer-diameter carbon nanotube.
Allowing their simulation to equilibrate at kelvin, Xu and Chen found that the water molecule sat at the center of the cage, but due to thermal agitation its orientation rattled about freely. Then they applied an electric field in the simulation, parallel to the nanotube.
Water molecules are electric dipoles: an excess of positive charge clings to the hydrogen atoms, balanced by a negative charge on the oxygen. An electric field permeating a volume of water applies a torque to the molecules, so that instead of moving completely at random they rock back and forth in a thermally driven motion known as libration. But because water molecules are electrically neutral overall, they do not move laterally in response to the field.
Confined in a cage, however, the water molecule behaved quite differently. Instead of undergoing libration, it settled quickly into a fixed alignment with respect to the field—the stronger the field, the smaller the angle between the water molecule and the field. The water molecule then spun around the field direction, like a tilted top. The orientation of the cage remained stable, but the whole structure— with spinning inside—moved through the confining nanotube, parallel to the field, even though the structure was electrically neutral. Odder still, this motion reversed direction when the field strength exceeded a critical value of about volts per angstrom.
To gain insight into this strange behavior, the theorists focused on the water molecule. Once the electric field was applied, any initial linear and rotational motions in the plane perpendicular to the field rapidly diminished as the water moved into its stable orientation. Xu and Chen explain that the energy and momentum of those initial motions has to go somewhere, and it ends up providing an impulse to linear motion along the field direction and rotation about that direction. Consistent with this reasoning, the linear velocity of the water molecule depended on its orientation at the moment when the field was turned on. The greater the change in alignment of the water in response to the field, the more energy was transformed into linear motion.
As for the change of direction when the field becomes stronger, Xu says that the interaction between the water molecule and the fullerene cage in the presence of an electric field is a complex problem that they are still working to understand. What’s important, he says, is that @ , unlike the plain fullerene, has a polarity that somehow allows it to be steered by an electric field, opening up novel nanotechnological applications.
Andrei Khlobystov of Nottingham University in the UK calls the work a “very exciting development … for accurate control of molecular motion” but admits that it is hard to understand. He suspects that the key to the puzzling behavior involves the fullerene’s modification of the normal interaction between water and an electric field. Khlobystov wonders whether similar behavior might be found for less exotic, and therefore more easily exploitable, molecules.
–David Lindley
David Lindley is a freelance science writer in Alexandria, Virginia.
References
- K. Kurotobi and Y. Murata, “A Single Molecule of Water Encapsulated in Fullerene C,” Science 333, 613 (2011)