A Breakthrough for Remote Lasing in Air
The nitrogen laser is probably the simplest laser scheme that can be implemented by amateurs [1]. Using normal air and a high-voltage pulse from a home-made electrode system, one can generate a laserlike emission, called “superfluorescence,” without the need for a carefully aligned cavity or any doped crystals or glasses. Strictly speaking, the superfluorescent radiation in air is not coherent and does not formally qualify as lasing, but the emission is directed and amplified, so this “air lasing” effect has been considered for remote atmospheric sensing and light-based radar (called LIDAR). In these applications, a laser pulse—in place of a high-voltage pulse—would generate the superfluorescence in the upper atmosphere or at a distant target. However, optically induced air lasing has proven to be much more difficult than the electrically based nitrogen laser. Years of research and the use of sophisticated laser-pump schemes have not produced enough air lasing for practical purposes. But now Alexandre Laurain and colleagues at the College of Optical Sciences, Arizona, have developed a novel and substantially more efficient way to obtain remote lasing in air [2]. Their technique is based on two steps: an infrared laser pulse first dissociates air molecules, and then an ultraviolet pulse excites the resulting atoms to energy levels that emit fluorescent radiation. This two-color approach leads to higher lasing output and can work with substantially lower input power than previous techniques using only a UV pulse.
One of the main motivations for developing remote air lasing is analyzing the chemical composition of the atmosphere over some large distance. In particular, “standoff” detection of trace gases would be invaluable for military and airport security, as well as environmental studies [3]. To identify target agents from a remote distance of a few meters to many kilometers, it is often practical to use a LIDAR (light detection and ranging) setup, in which light is directed at the target and scattered back to a detector located next to the original source. In the most straightforward case, the laser excites the target agent to a fluorescent state and the fluorescent, backscattered emission is detected. However, reliable identification becomes harder with greater distance, as optically excited fluorescence is isotropically emitted. This means that the number of photons reaching the detector scales with the inverse square of the distance, so for low concentrations of remote target agents, the detectable fluorescent signature quickly falls below the single-photon limit. Air lasing offers a possible solution to this problem. A laser would be directed into the atmosphere, where it generates an “air laser” that emits in both the forward and backward directions. The signature of molecules along the path of the backward-propagating laser beam would be identified through their characteristic absorption.
Optically induced air lasing was demonstrated more than a decade ago [4]. Previously reported approaches have relied heavily on filamentation, i.e., a phenomenon that keeps light confined to a narrow channel that results from a balance between the nonlinear optical effects of self-focusing and plasma formation. Filaments can be thought of as light-induced plasma channels, with a resulting central depression of the refractive index that guides light similar to an optical fiber [5]. The first air lasing schemes used ultraviolet filamentation, in which air molecules within the filament were excited by the UV light into a fluorescence-emitting state. Subsequent work has refined the technique over the past few years, but the reported backward emission has proven rather weak so far, requiring the use of highly sensitive photomultipliers in the detection. A more convincing lab demonstration of air lasing was reported in 2012 by a group using midinfrared filamentation with a -micrometer ( ) laser, which led to a dramatic enhancement of nitrogen lasing compared to other excitation wavelengths [6]. However, these experiments required high-pressure nitrogen and could not be extended to atmospheric air. Following up on the earlier UV filamentation experiments, Laurain et al. now propose a radically new two-color excitation scheme and demonstrate its suitability to significantly enhance the laser gain in both nitrogen and oxygen [2].
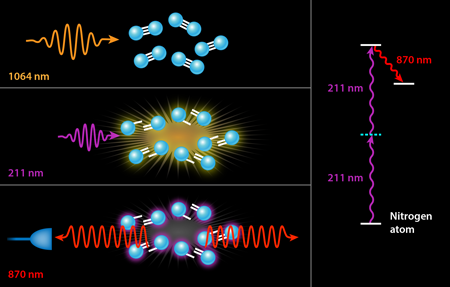
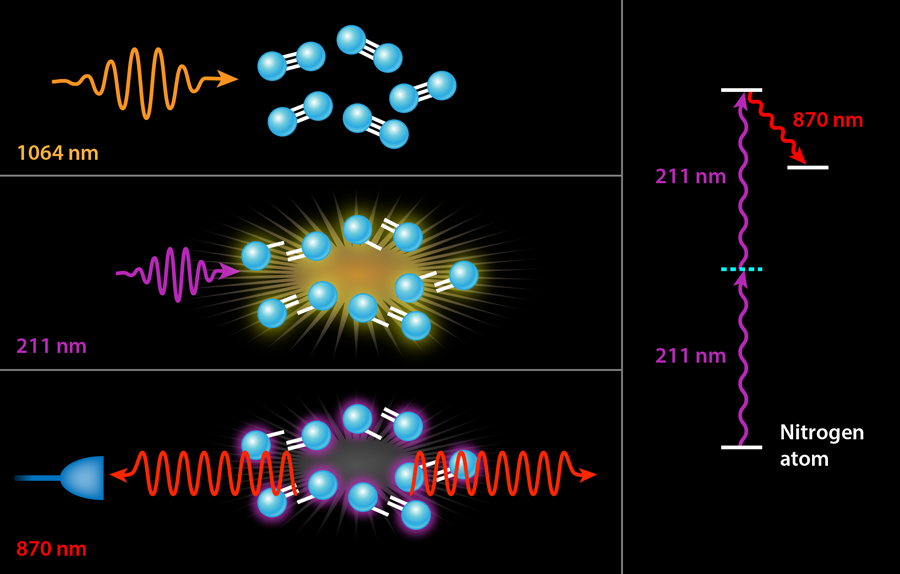
In previous experiments, the ultraviolet pump served two different purposes simultaneously. The first was dissociation, i.e., the breaking apart of the nitrogen or oxygen molecules into their constituent atoms. The second was pumping the resulting atoms into their upper laser state by a two-photon excitation scheme. It is only this second step that strictly requires a deep UV laser with a wavelength tuned to the narrow transition of nitrogen at nanometers (nm). The generation of - light generally involves three nonlinear conversion steps, which is quite costly in terms of efficiency: It takes joules of input energy to obtain a few millijoules ( ) of laser light at . While the exact wavelength of these photons is only important to pump the laser process in nitrogen, it would be far more efficient to separate the two steps, using infrared laser light (which is already available as the “seed” light to the ultraviolet laser conversion process) to dissociate the molecules via multiphoton processes in an infrared filament. This is the approach followed by Laurain et al., who use - pulses of - laser light to dissociate a sample of atmospheric air. They subsequently fire a pulse of ultraviolet light at the appropriate wavelength for pumping either the nitrogen or oxygen atoms (see Fig. 1).
Using this novel two-color scheme, Laurain et al. manage to observe nanojoules of backward lasing in atmospheric nitrogen—an enhancement by a factor of over previous nitrogen-lasing experiments using an otherwise identical pump energy at . A smaller threefold enhancement is seen in atmospheric oxygen with a pump at . But more importantly, both lasing processes appear to be nearly threshold-less with the two-color scheme, i.e., the researchers observed backward lasing emission even as they lowered their ultraviolet pump energy to well below microjoules. By contrast, previous air lasing demonstrations measured no lasing below a threshold of a few millijoules of ultraviolet pump energy. It should be noted, of course, that the scheme of Laurain et al. also requires hundreds of millijoules of infrared photons, but these are significantly less expensive to produce than ultraviolet photons.
While the results have been obtained in lab experiments, the authors extrapolate that they may be able to induce air lasing at distances greater than meters. Assuming this can be experimentally verified, it would still be short of the kilometer ranges necessary for trace gas detection in the upper atmosphere. Nevertheless, the demonstration of Laurain et al. constitutes a major step ahead in that direction. Even if air lasing is limited to only tens or hundreds of meters, it would open up a plethora of new applications in standoff spectroscopy. A potentially interesting avenue to pursue would be to combine ultraviolet excitation schemes with the midinfrared sources from experiments like those in Ref. [6]. Such an exotic “color” combination of extreme ultraviolet and similarly extreme infrared radiation may enable standoff spectroscopy at atmospheric distances.
Editors’ note: After publication of this article, we became aware of a series of papers demonstrating that a two-color approach, similar to that described in this article, enhances emission in a backwards lasing process. On p. 444 of Science 331, 442 (2011), Dogariu et al. show that a pre-pulse with 532-nanometer wavelength dissociates oxygen molecules in air and state that this pre-pulse “enhances the coherent backscattered emission by a factor of .” Dogariu et al. reported a similar measurement in the conference proceedings: CLEO:2011 - Laser Applications to Photonic Applications, OSA Technical Digest Optical Society of America, 2011. Additionally, the same authors showed in Figure 3d of the conference proceedings Imaging and Applied Optics 2014, OSA Technical Digest Optical Society of America, 2014, paper LW2D.3 that a pre-pulse, which dissociates nitrogen in air, enhances the intensity of emission by a factor of 500.
References
- C. L. Stong, “An Unusual Kind of Gas Laser that Puts Out Pulses in the Ultraviolet,” Sci. Am. 230, 122 (1974)
- Alexandre Laurain, Maik Scheller, and Pavel Polynkin, “Low-Threshold Bidirectional Air Lasing,” Phys. Rev. Lett. 113, 253901 (2014)
- P. R. Hemmer, R. B. Miles, P. Polynkin, T. Siebert, A.V. Sokolov, P. Sprangle, and M. O. Scully, “Standoff Spectroscopy via Remote Generation of a Backward-Propagating Laser Beam,” Proc. Natl. Acad. Sci. U.S.A. 108, 3130 (2011)
- Q. Luo, W. Liu, and S. L. Chin, “Lasing Action in Air Induced by Ultra-Fast Laser Filamentation,” Appl. Phys. B 76, 337 (2003)
- L. Bergé, S. Skupin, R. Nuter, J. Kasparian, and J.-P. Wolf, “Ultrashort Filaments of Light in Weakly Ionized, Optically Transparent Media,” Rep. Prog. Phys. 70, 1633 (2007)
- D. Kartashov, S. Ališauskas, G. Andriukaitis, A. Pugžlys, M. Schneider, A. Zheltikov, S. L. Chin, and A. Baltuška, “Free-Space Nitrogen Gas Laser Driven by a Femtosecond Filament,” Phys. Rev. A 86, 033831 (2012)