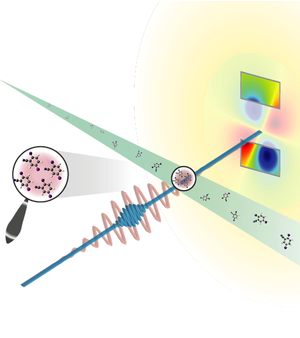
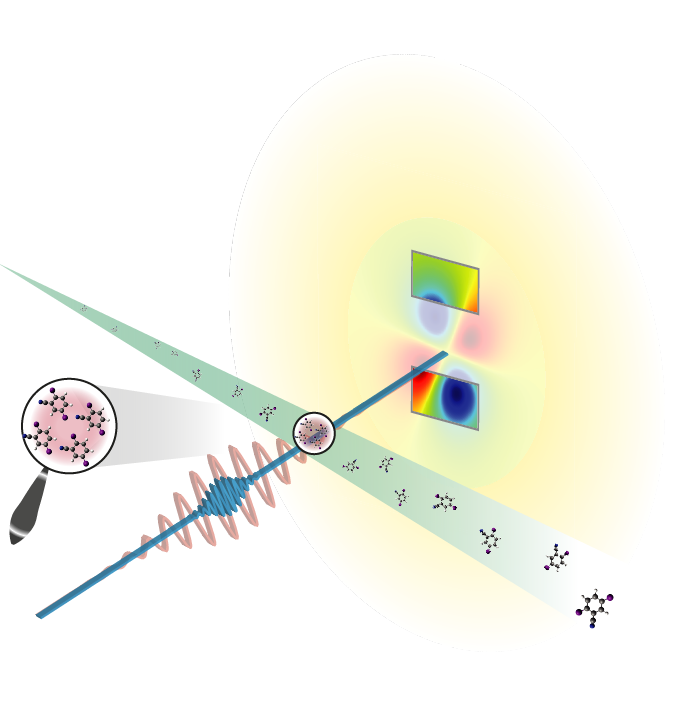
X rays Measure Lone Molecules
A classic way to measure the atomic structure of a molecule is to scatter x rays from a crystal, but crystals are sometimes impossible to make. Now researchers reporting in Physical Review Letters have used x-ray scattering to measure the distance between two atoms in a molecule by using a large number of aligned, identical molecules in a molecular beam. Although the distance was already better known from other techniques, the experiment shows the feasibility of using extremely bright x-ray sources to explore previously inaccessible phenomena in isolated molecules.
Of the many ways of measuring atomic-scale distances, x-ray diffraction has been one of the most precise techniques for a century. And it remains the most flexible and powerful way to determine molecular structure, for example of proteins, molecules that often have thousands of atoms. Usually you need to coax many copies of a molecule to nestle together to form an orderly crystal before you can use x rays to learn the atomic structure. X rays scatter, or “diffract,” strongly from a crystal only in certain special directions, and researchers use the angles of these bright spots to calculate the atomic positions. Large, defect-free crystals are critical for bright diffraction spots, but for many proteins, it is difficult to grow such crystals.
However, even small crystals can work well with superbright x-ray pulses from one of the new free-electron lasers, such as the Linear Coherent Light Source (LCLS) at the SLAC National Accelerator Laboratory in California, which began operation in 2009. Researchers have used such sources to analyze tiny crystals with just tens or hundreds of protein molecules. “Experimentally it’s a big step forward, but conceptually it’s still crystallography,” says Jochen Küpper of the Center for Free-Electron Laser Science (CFEL) in Hamburg, Germany. Now he and a large, international team have taken the next step and worked with isolated molecules, rather than a crystal, to measure an atomic distance.
The researchers used a beam of a small molecule called diiodobenzonitrile (DIBN), which is a modified six-carbon ring (benzene) with iodine atoms attached at two opposite ends of the ring. The two iodines have many more electrons than the other atoms, so, when hit with x rays, they generate most of the scattering. In addition, the team used a standard laser to align the iodine-iodine axis with the laser’s electric field in advance of the x-ray pulse. Researchers have lined up molecules in this way before, says Küpper, but the team’s experiment set out to show that such a system could also produce a measurable diffraction signal.
The team set up their alignment apparatus at LCLS and collected x-ray scattering data in 2010, and they are now publishing their analysis of the results. Each x-ray pulse hit about a hundred molecules, but it still took hundreds of thousands of pulses to accumulate enough x-ray scattering data. Instead of the sharp peaks seen for crystals, the scattering intensity smoothly increased with the scattering angle over the range they measured. Based on the shape of this scattering curve, the team deduced a distance of about 800 picometers between the iodine atoms, slightly larger than the known value of about 700 picometers.
“We could have determined that distance better by other methods, no question,” says Küpper. Moreover, the shortest x-ray wavelength available in 2010, about picometers, was almost as big as the distance measured. The latest facilities provide wavelengths closer to picometers, as well as more frequent pulses, either of which would provide a clearer measurement. He also notes that the current generation of machines could be used to track atomic positions during a chemical reaction on the femtosecond time scale.
“We knew this was possible. This experiment shows that it’s feasible,” says Nora Berrah of the University of Connecticut in Storrs. She says the work raises researchers’ confidence that new capabilities—including a detector her team recently provided for LCLS and an upcoming source at CFEL—will allow free-electron lasers to produce genuinely unexpected results.
–Don Monroe
Don Monroe is a freelance science writer in Murray Hill, New Jersey.