Dipolar Gas Chilled to Near Zero
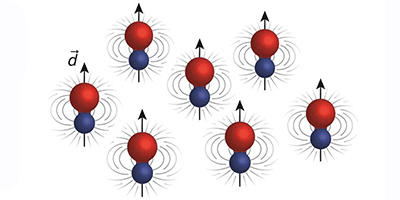
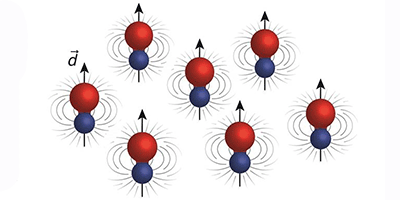
Cooling dipolar molecules to near absolute zero could produce new quantum states of matter, as the dipoles exert strong long-range forces on each other that are not found in nature. Researchers have now chilled a gas of sodium-potassium (NaK) molecules in their absolute ground state to microkelvin temperatures. The NaK molecules have a stronger dipole interaction and are more stable than previous superchilled molecules, which makes them ideal for exploring the impact of dipolar interactions in the quantum regime.
Molecules differ from atoms in that they rotate and vibrate. These extra degrees of freedom complicate efforts to cool molecules with traditional laser techniques. To circumvent these challenges, researchers have recently been able to form certain ultracold molecules—the fermionic KRb and the bosonic RbCs—directly out of a gas of ultracold atoms, and to subsequently transfer them into their rovibrational ground state.
Martin Zwierlein and his colleagues at MIT have added the fermionic NaK to this small class of ultracold molecules. The team started with cold sodium and potassium atoms and then used a magnetic field to access a so-called Feshbach resonance that combines the atoms into weakly bound molecules. Subsequently, a pair of lasers couples this Feshbach state to the rovibrational ground state, allowing a smooth transfer to the lowest energy state without adding kinetic energy to the gas. Because NaK doesn’t easily dissociate, the team found it has a relatively long lifetime (greater than 2.5 seconds), which benefits future experiments. If the temperature can be lowered by another order of magnitude, the NaK gas will enter the quantum degenerate regime where exotic forms of matter are predicted, such as a topological superfluid that contains Majorana fermions or a dipolar quantum crystal that could be solid and superfluid at the same time.
–Michael Schirber
This research is published in Physical Review Letters.