Birth of a Crystal
World War I may have been inevitable, but it was a single, surprising event–the assassination of Archduke Ferdinand–that finally precipitated it. On a more humble level, the crystallization of ordinary salt from salt water is a major molecular rearrangement that is also triggered by a single, unpredictable event. The trigger is so exceedingly rare that even powerful supercomputers haven’t been able to identify the first stages of crystallization. But by using a recently developed scheme to pick out such rare events, a paper in the 30 January PRL reports the first computer simulations giving detailed structures of what appear to be nascent sodium chloride crystals.
To start a crystal forming in salt water, a sodium ion (charged atom) and a chloride ion must find one another. Like future lovers at a crowded party, they can’t connect unless the hordes between them–water molecules–shift around in a way that lets the pair come together. But with water molecules packed tightly around each of them, the odds for a meeting are extremely low. Standard computer simulations cannot run long enough to wait for such unlikely events, so researchers have few detailed pictures of the early stages of crystallization from solution. The problem is that it’s hard to come up with the exact sequence of water molecule motions that would bring the two atoms together.
Building on a technique developed by others, Dirk Zahn, of the Max Planck Institute for the Chemical Physics of Solids in Dresden, Germany, used a trick to find these promising sets of motions. He first told his computer to drastically reduce the attraction between the ions and water, which allowed the ions to find one another more easily, at least in some runs. He then chose one of those “artificial” runs, which was essentially a movie showing a sequence of water molecule and ion motions that appeared to lead toward crystallization. His final step was to reset the attraction to the normal strength and alter the movie bit by bit to find sequences that were relatively probable, given the temperature and other conditions.
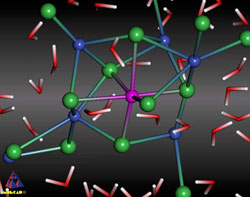
Zahn then inspected the final frames of 20 of these movies that showed hints of crystallization. While the molecular arrangements differed in detail, most contained a sodium ion surrounded by six chloride ions forming a crude octahedron, similar to the arrangement in a sodium chloride crystal. He found no similar structures centered on chloride ions. Although these appear to be the beginnings of crystals, Zahn cautions that much longer simulations would be needed to determine whether they eventually become true crystals rather than dissolving away. Still, no other simulations had gotten even this far.
Jay Rasaiah of the University of Maine in Orono says that before this work “we knew nothing about the mechanism of aggregation,” but he calls the octahedral structure “a very suggestive mechanism.” David Chandler of the University of California at Berkeley, whose group developed the technique upon which Zahn’s was based, is also impressed. “This is the kind of thing [our technique] was invented for, and he’s done a neat job.”
–Don Monroe
Don Monroe is a freelance science writer in Murray Hill, New Jersey.


