Tuning the Sun’s Rays
German researchers have boosted the frequency of sunlight, effectively making blue light from green. So-called up-conversion is commonly done with bright laser sources but never before with random sun rays. The process, described in the 6 October PRL, could benefit solar energy technology, as it would allow low frequency sunlight to be “recycled” into a higher frequency range, where some solar panels are more sensitive.
Many natural materials exhibit fluorescence in which they down-convert incoming light to lower frequencies. Up-conversion is rarer, typically requiring two or more low frequency photons to be absorbed by a single molecule. The combined photon energy knocks one of the molecule’s electrons into an upper energy level, and it later falls to the ground state, emitting a single high frequency photon with double the energy of any of the incoming ones. The multiple absorptions must occur nearly simultaneously, so this process requires a high concentration of photons. Only lasers can provide the necessary intensity of roughly a million times sunlight, says Stanislav Baluschev of the Max-Planck Institute for Polymer Research in Mainz, Germany.
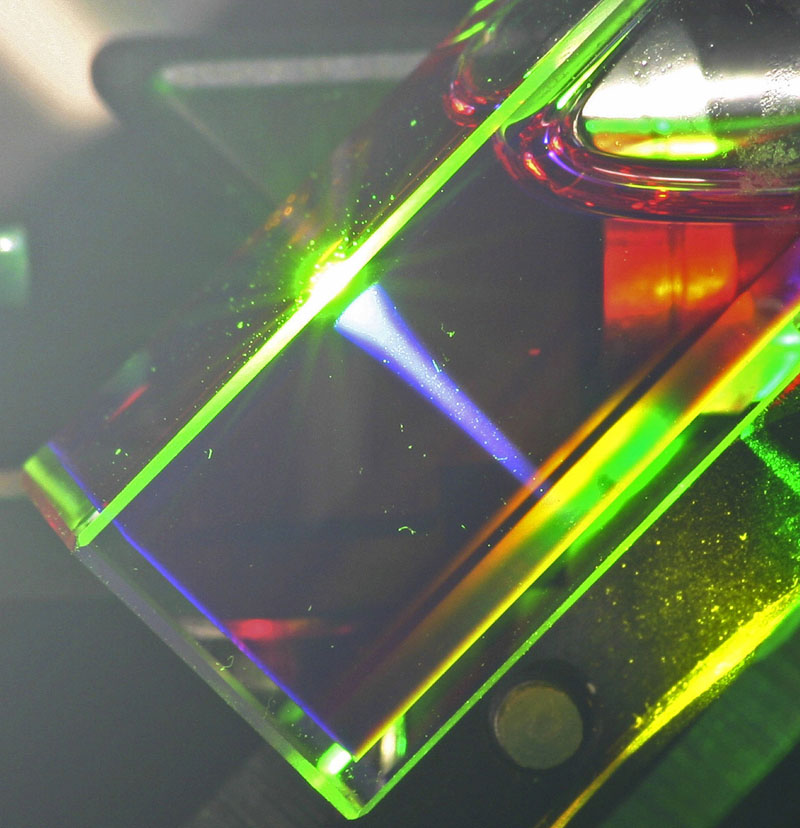
Baluschev and his colleagues have developed an alternative technique for up-conversion that can work at much lower intensities. Instead of adding photons in a single molecule, the researchers use two molecules, each storing one photon’s worth of energy for later summation. In previous work, the team showed that their mechanism can up-convert laser light, but they have optimized it now for ordinary, random light sources. In a demonstration with sunlight, which was filtered of all but its green frequencies and focused so that its intensity was 100 times higher than normal, the researchers recorded a blue shaft of light in their liquid up-converter.
To achieve this, the team mixed a green-absorbing “sensitizer” with a blue-emitting polymer in a solution. The sensitizer was a ring-shaped molecule with a palladium atom core that can lock up absorbed photon energy in a long-lived excited state called a triplet. Through a poorly understood mechanism, some of these sensitizer triplets can transfer their energy to emitter molecules, thereby generating even longer-lasting emitter triplets. “They live for about 5 milliseconds, which is forever compared to other excited states,” Baluschev says. If two of these emitter triplets interact with each other in the solution, one triplet can steal the energy of the other to elevate one of its electrons into a doubly-excited state. From there, the emitter drops back to its ground state with the release of a blue photon. In effect, the energy of two incoming photons is added together with the help of these molecular middlemen.
The researchers determined the maximum efficiency of their system to be 1% (one blue photon out for every 100 green photons in). This may seem small, but solar cells are only sensitive to a portion of the sun’s spectrum. So up-conversion could capture unused photons to make useable ones. “It’s an extra channel for harvesting the energy from the sun,” says Panagiotis Keivanidis of the University of Cambridge, England. Jan Goldschmidt of the Fraunhofer Institute for Solar Energy Systems in Freiburg, Germany, agrees but says that taking green to blue only benefits certain organic solar panels. “To be relevant for the dominant silicon solar cell technology, the absorption range has to be shifted to the infrared,” he says. Baluschev says his group is currently working on other combinations of molecules that can up-convert at lower frequencies.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.