The Little Bubbles that Could
An air bubble will live peacefully on the side of your glass of water for a long time, but shrink that bubble to the nanoscale, and the overwhelming force of surface tension should prevent it from ever forming. Yet researchers have been observing them for several years, thanks to recent advances in imaging technology. The puzzle of nanobubbles has deepened with the 18 May Physical Review Letters, in which a team reports that nanobubbles can withstand shock waves exerting wide swings in pressure, a sign of what they call superstability. Aside from any impact on solving the riddle, the results suggest that tiny bubbles could be useful pieces of nanotechnology, perhaps serving to protect delicate machinery in future biochemical devices.
Surface tension, the force that makes water bead up on a freshly waxed car hood, also keeps a submerged air bubble as round as possible. Surface tension pushes in on the bubble, and the smaller the bubble, the higher the air pressure inside the bubble must be to keep it from collapsing. For a 100-nanometer-diameter bubble, the pressure should theoretically be at least five times that of the atmosphere, easily high enough to force the gas to dissolve into the surrounding liquid. Blip, the bubble should disappear. But it doesn’t.
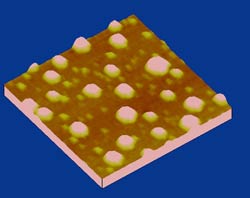
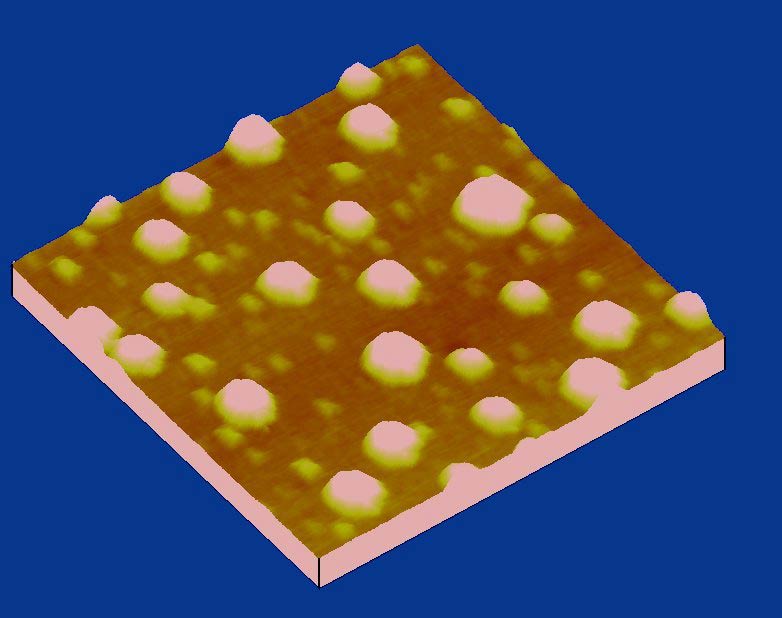
In 2000 Japanese researchers discovered these surprisingly small bubbles on a silicon surface covered with water [1]. They were using the new imaging technology called “tapping mode” atomic force microscopy (AFM), where a tiny lever vibrates up and down to tap the sample. In these and many other experiments, nanobubbles behave differently than ordinary bubbles, says Andrea Prosperetti, of Johns Hopkins University in Baltimore. Researchers still don’t agree on how nanobubbles survive, although they have proposed several different theories.
Detlef Lohse of the University of Twente in the Netherlands and his colleagues decided to check the bubbles’ ultimate stability by subjecting them to severe stress. They created nanobubbles in a drop of water on a silicon wafer coated with a hydrophobic material and imaged it with AFM for the “before” picture. Next they submerged the wafer in a water bath and blasted it with a powerful, 6-microsecond-long shock wave produced by the same kind of generator that can destroy kidney stones. Each pulse included positive and negative pressures over 60 atmospheres. This level of abuse would ordinarily create a drastic inflation of bubbles, in a process called cavitation, but the “after” image of the nanobubbles showed them to be unaffected. Based on the bubbles surprising survival, the team dubbed them “superstable.”
This stability could be useful, says team member Bram Borkent of the University of Twente. Chemical analysis devices now under development will need to move fluid through nano-channels cut into solids, but pushing the fluid through such tiny spaces is hard. “If you can create these bubbles on purpose then you can cover a nanochannel with bubbles,” which might reduce the friction, says Borkent. Other researchers envision using nanobubbles as computer memory structures or to remove blood clots. “Wherever surface effects are important,†Borkent says, “nanobubbles may play an important role.”
Prosperetti sees the new work as part of a larger story. “It is another tantalizing example of the surprises that lie in the gray area of nano–the not-so-small to be quantum, but not-so-large to be fully macroscopic.”
–Mike Wofsey
References
- N. Ishida, T. Inoue, M. Miyahara, and K. Higashitani, “Nano Bubbles on a Hydrophobic Surface in Water Observed by Tapping-Mode Atomic Force Microscopy,” Langmuir 16, 6377 (2000)