Clarity through Diversity
Milky-white cataracts, the world’s leading cause of blindness, can occur when proteins in the lens of the eye aggregate into clumps. In an upcoming issue of Physical Review Letters, researchers report that although one pure lens protein forms clumps, a mixture of that same protein with another lens protein is less clumpy. By comparing experiments with extensive computer simulations, they conclude that the effect arises because the two proteins attract each other, but not too strongly. The new information on lens protein interactions could have benefits beyond cataract research because controlling similar attractions could help keep particles dispersed in material and food processing.
The lens of the eye consists of an ordered array of cells, filled mostly with water and several proteins called crystallins. The lens is clear only when the different protein types are evenly dispersed. But if they clump up or segregate from one another by type, a cataract can result because the clumps scatter light, somewhat like the droplets of condensed water that make up clouds.
For decades, researchers have studied the segregation and aggregation properties of naturally occurring mixtures of lens crystallins and of single components, but no one has looked at just two crystallins at the high concentrations that occur in the eye. A Swiss and American collaboration led by Peter Schurtenberger of the University of Fribourg in Switzerland has now explored the behavior of two of the main crystallins, using neutron scattering to probe their detailed structure. First the team looked at each protein on its own. They confirmed previous findings that the larger of the two acts just like a collection of 18-nanometer-diameter billiard balls, but the smaller molecules, only 3 or 4 nanometers in diameter, tend to stick together, forming light-scattering clumps. But when the researchers mixed the two, the clumping was reduced.
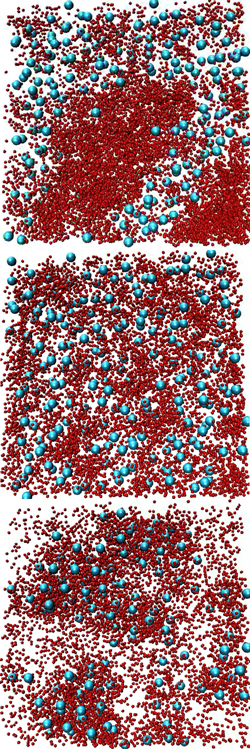
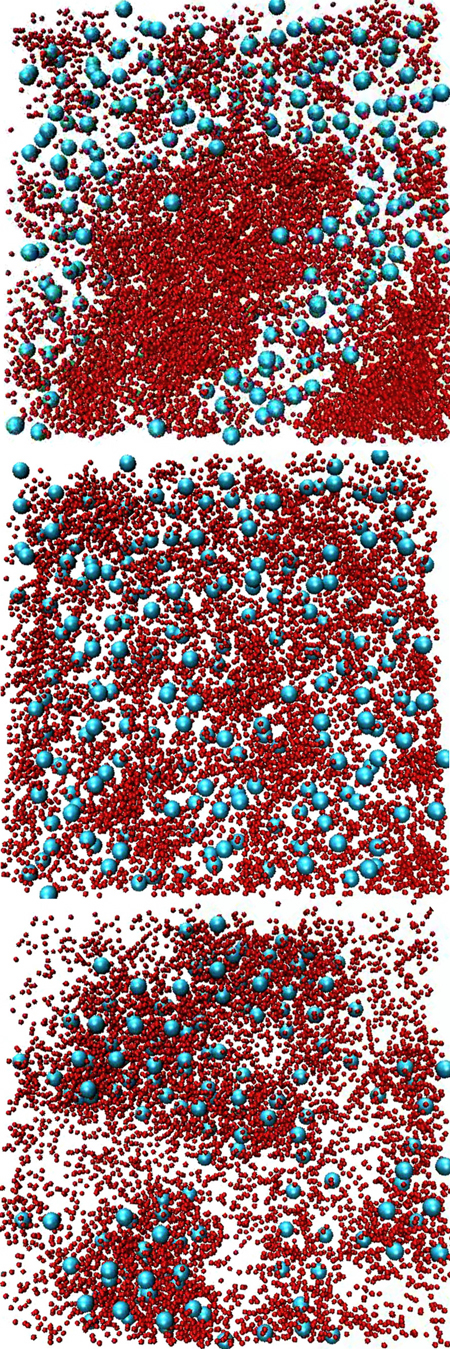
To investigate why the larger crystallin reduced clumping of the smaller one, the team performed large-scale simulations using a group of networked computers. Guided by the known single-molecule behavior, they used tens of thousands of hard spheres to represent the protein molecules. They mixed spheres with two different diameters and made the smaller ones sticky. The team then varied the attraction between the large and small proteins. With no attraction, an initially uniform mixture separated into regions that were rich in either large or small proteins, in disagreement with the original lab experiment.
But adding a small attraction between the large and small proteins kept the two species thoroughly mixed. Moreover, the detailed structure in the simulation agreed with that observed by neutron scattering. “We can overcome [the segregation] by turning on a little bit of an attraction between the small and the big ones,” says Schurtenberger. But if the researchers made the simulated attraction even a little stronger, both small and large proteins started to bind together in large clusters. “The stability that we find comes from a finely-tuned balance,” he says, suggesting that a similar balance may be needed for healthy lenses.
In the long run, researchers hope that modifying the protein interactions could prevent cataracts. But Schurtenberger also notes that tuning intermolecular attractions could help maintain uniformity in food processing and in advanced materials used to make optical fibers.
Annette Tardieu of the University of Paris 6 and the National Center for Scientific Research (CNRS) in France says that such a crystallin mixture “has never been studied before in detail” and may well give insight into cataracts. But she is not convinced of the role of the attractive force, because the hard spheres used in the simulations could mask other important details of real proteins. “I would prefer to trust the experiments,” she says.
–Don Monroe
Don Monroe is a freelance science writer in Murray Hill, New Jersey.