Sorting Blood Cells via Their Stiffness
The onset of diabetes, sickle-cell anemia, and malaria are all marked by a gradual stiffening of red blood cells. While devices and methods exist for sorting blood cells by size, those techniques are insensitive to other properties such as a cell’s stiffness. Now, Dmitry Fedosov and colleagues at the Jülich Research Center in Germany have used computer simulations to show how modifications to a microfluidic cell-sorting device could allow red blood cells to be sorted via their deformability. Such devices could allow clinicians to quickly and cheaply spot the onset of diseases that involve cell stiffening.
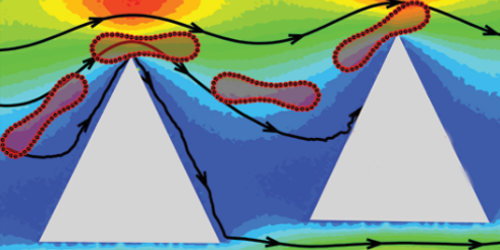
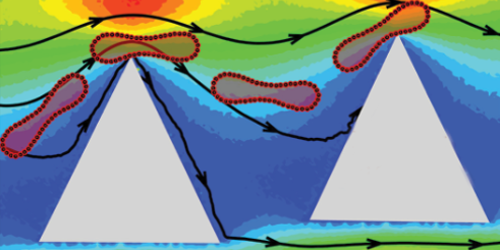
The investigated cell-sorting device normally consists of staggered rows of micrometer-sized circular posts that organize a flow of cells into different streams according to cell size. Fedosov’s team has shown that if the posts instead have sharp edges, then the device could separate cells into groups with differing stiffnesses. Their simulations show that a squishy red blood cell bends when it bumps into a diamond- or triangular-shaped post, and this bending causes the cell to hug the post and stay on course. In contrast, an unbending stiff cell bounces off any post it hits and is knocked into an adjacent stream. As the cells encounter successive rows of posts, they slowly fan out according to their stiffness. Simulations with circular posts show no such separation.
The team says that there are no major hurdles to making their proposed device, and other researchers have sorted cells experimentally using noncircular pillars. However, the team adds that the optimal experimental settings—such as cell flow rate—needed to sort cells with varying degrees of stiffness will require fine-tuning. While the team has so far simulated only red blood cells, the technique should work on any type of cell.
This research is published in Physical Review Fluids.
–Christopher Crockett
Christopher Crockett is a freelance writer based in Arlington, Virginia.