Playing pool with atoms
One of the most basic ways to learn about few-body interactions in an atom is to break it apart. Understanding this atomic fragmentation is most challenging when the particles emerge with little kinetic energy. Since perturbation theory cannot be used, all of the particles need to be treated on an equal footing, and the behavior of the system at long times and large distances becomes crucial.
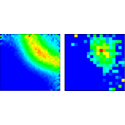
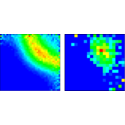
For interactions that fall off as or slower—such as that between charged particles—large-distance correlations subtly influence the breakup, but exactly how this occurs has been a subject of controversy for more than 50 years. Writing in Physical Review Letters, Xueguang Ren, Alexander Dorn, and Joachim Ullrich of the Max Planck Institute for Nuclear Physics in Heidelberg use electron bombardment to fully ionize helium at an excess energy of only 5 eV. They find the electrons—two from the helium atom plus the projectile electron—emerge predominantly in the configuration of an equilateral triangle, in accordance with the basic prediction of so-called Wannier-type threshold laws. But they also show that on a more detailed level some unexpected structure persists, indicating that the fragmentation dynamics at these energies is more subtle than the simple Wannier picture would suggest.
The paper is an important step forward in the understanding of threshold fragmentation, but it also raises new questions by showing unexpected complexity in the details of the atomic breakup. – Thomas Pattard