Physicists Needed to Help Advance Cancer Treatment
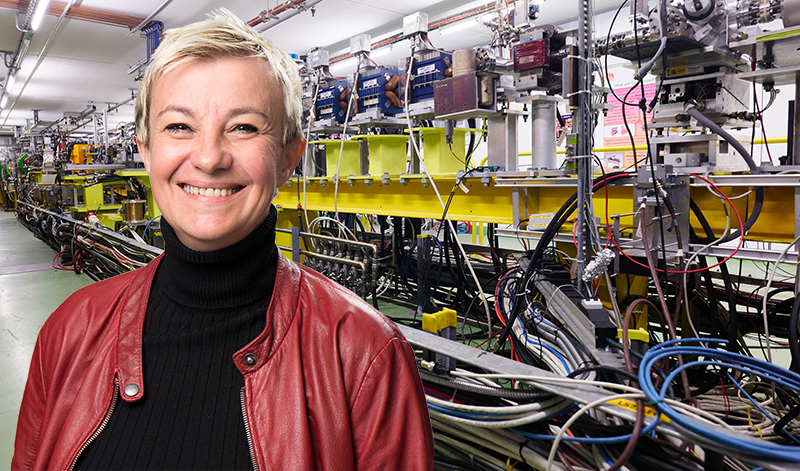
To kill a malignant tumor, clinicians typically blast it with some form of radiation. This treatment is tailored to an individual’s needs, allowing clinicians to enhance the therapeutic impact of the radiation while limiting its side effects. “The goal is to cure a person’s cancer without any toxicity to surrounding healthy tissues,” says Marie-Catherine Vozenin, a radiation biologist at the University Hospital of Geneva.
The past 70 years have seen significant advances in targeted radiation therapies. Today, clinicians can focus the x-ray, electron, or proton beams they use more precisely than ever before, reducing damage to surrounding tissues and organs. But there is still scope for improvement. Further advances will require upgrading the machinery currently used in hospital settings, says Vozenin, who has spent her career studying how different forms of radiation interact with healthy and cancerous cells.
In a new review, Vozenin and her colleagues outline the clinical and technological steps needed to take a promising new radiation-therapy method called FLASH from accelerator-based facilities—where it has so far been tested—into hospitals [1]. Vozenin spoke to Physics Magazine about the open problems in developing new radiation therapies and why solving them cannot be done by clinicians alone.
All interviews are edited for brevity and clarity.
What got you interested in studying the effects of radiation on the human body?
Worldwide, more than 50% of the 20 million people diagnosed with cancer each year will undergo some form of radiation therapy as part of their treatment. That’s a huge number of people. Many more will be exposed to radiation through routine or diagnostic imaging procedures from mammograms of breast tissues to x rays of broken bones. It is therefore important to understand how to protect normal tissues from this radiation. We also need to work toward enhancing the therapeutic impact of radiation treatment, so we can limit unnecessary exposure. That is why we are developing “FLASH” radiotherapy.
What is FLASH radiotherapy?
It’s a high-dose therapy that delivers radiation to a cancerous tumor in a fraction of a second. In conventional techniques, patients receive the required radiation over a few minutes. FLASH techniques provide the same dose but over a few milliseconds or less.
What benefits does this shorter time frame offer?
For in vivo animal models we have shown that shorter-duration exposures are less likely to damage normal tissue. Studies so far indicate that this reduced damage arises because the normal tissue behaves as if it hasn’t seen the beam. Meanwhile, the tumor responds as it would for longer-duration exposures.
What makes normal tissue “blind” to shorter-duration exposures?
That’s the million-dollar question we are trying to answer. Several hypotheses have been proposed. But so far, they have all been proved wrong.
To try to solve the puzzle, we initially probed what happens at the physical-chemical level when beams of different types and duration interact with normal and cancerous tissues. We found no differences between the behavior of the two tissue types. Now we are digging deeper and looking at what happens at the biological level to see if we can find the solution.
That seems like a problem for biologists to solve, where does the physics come in?
For FLASH therapies to move from in vivo animal tests to fully-fledged treatments for humans, we need to reduce the size of the accelerators from hundreds of meters in length (as found at CERN and other research facilities) to a few meters in length (as found in hospitals). Those smaller accelerators, however, can’t currently produce protons, electrons, or x rays at the dose rates we need for FLASH. We really need physicists and engineers to help develop technologies that can meet these goals.
What kind of dose-rate increase is required?
Currently, radiation therapies are done with doses of around 4 grays per minute, where a gray is equivalent to 1 joule of energy being absorbed by 1 kilogram of tissue. For FLASH therapies the minimum dose rate we need is 100 grays per second. That’s a huge increase. But I think that with new or updated accelerators, we can get there.
The cost of chemotherapy drugs for patients is huge. If we can develop an x-ray FLASH accelerator that fits in a standard hospital facility, for example, we could have more effective treatments that are less toxic to patients and that most people could afford. That would be an amazing achievement.
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- M.-C. Vozenin et al., “FLASH: New intersection of physics, chemistry, biology, and cancer medicine,” Rev. Mod. Phys. 96, 035002 (2024).