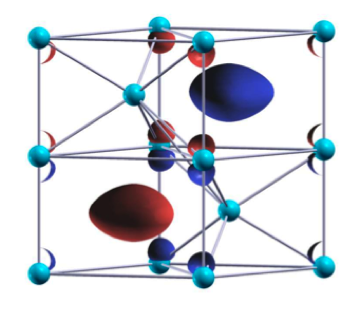
Pressure dulls shiny metals
High pressure can radically change the behavior of simple substances. Consider sodium. At ambient pressures it has a symmetric crystal structure. Under large enough pressure, it changes from a shiny metal to a transparent insulator with several low-symmetry crystal structures. At very high pressures, the core electrons overlap and, because of the Pauli exclusion principle, give rise to less symmetric charge distributions. Coulomb repulsion between core and valence electrons localize charge in interstitial positions instead of at the nuclei, eventually leading to an insulating state.
In a paper published in Physical Review Letters, Matteo Gatti, Ilya Tokatly, and Angel Rubio, from Universidad del País Vasco and the Basque Foundation for Science, both in Spain, and the Fritz-Haber-Institut der Max-Planck-Gesellschaft, Germany, use first-principles calculations to analyze the optical response of sodium under high pressure. They find that an unusual kind of electron-hole pair—an exciton—rises from the interstitial charge localization. The authors predict that at pressures above the metal-insulator transition, sodium should be transparent in one polarization direction but reflective, like a normal metal, in the other. Photoluminescence experiments along the two axes of polarization should elucidate the nature of the bound exciton as well as the crystal structure at high pressures. – Daniel Ucko