How to Create a Ghost Chemical Bond
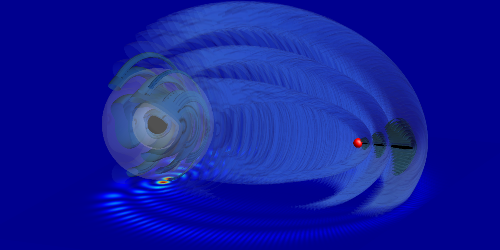
It goes without saying that a chemical bond requires, at the bare minimum, two consenting atoms. But a proposed experiment might reduce that requirement to just one, providing researchers with a new perspective on unusual chemical bonds. Matthew Eiles and colleagues at Purdue University in West Lafayette, Indiana, have come up with a way to construct a so-called trilobite bond—named after the electronic wave function’s resemblance to fossils of the long-extinct arthropod—by carefully manipulating a Rydberg atom, an atom with one electron in a highly excited state.
Normally, scientists have observed trilobite bonds in special types of diatomic molecules, such as and . In these cases, one of the atoms is in a Rydberg state, while the other is in its ground state. Because the Rydberg’s pumped-up outer electron occupies a very distant orbital, these “trilobite molecules” are unusually large, about 1000 times larger than typical diatomic molecules. Using numerical analyses, Eiles and colleagues show that through a precise sequence of alternating electric and magnetic field pulses, the electronic wave function of a Rydberg hydrogen atom can be sculpted to match that of a trilobite molecule. This leaves the excited electron strongly localized to a point in space, dozens of nanometers from the nucleus. The wave function should persist for at least 200 , in effect temporarily bonding the Rydberg atom to a nonexistent “ghost” atom.
Experimentalists will need to figure out how to accommodate the stringent requirements for synchronizing the pulses and blocking external fields. If these hurdles could be overcome and a ghost bond is produced, the system could be observed via electron- or x-ray-scattering experiments. While applications are speculative, the team imagines that it might be possible to see if such a preformed bond modifies chemical reaction rates in some way.
This research is published in Physical Review Letters.
–Christopher Crockett
Christopher Crockett is a freelance writer based in Arlington, Virginia.